February 2001, Vol 23, No. 2 |
Update Articles
|
Food induced asthma attacks in childrenD K K Ng, K B Tang HK Pract 2001;23:52-55 Summary Asthma is a common chronic illness in childhood. It is common belief that certain kinds of food trigger attacks or exacerbation of asthma. However, the actual documented prevalence of food induced asthma is less than the perceived prevalence. It is important for medical practitioners to firmly establish the diagnosis of food-induced asthma before advising a patient to avoid a specific food. Introduction Asthma is defined as episodic wheeze and/or cough that occurs in a clinical setting where asthma is likely, and in the absence of other mimicking conditions.1 Studies from the United Kingdom,2-6 Australia,7-8 New Zealand,9,10 Sweden,11 Finland,12 the United States13 and Canada14 showed evidence of an increase in the prevalence of asthma in children and young adults over the last two decades. A similar increase has also been reported in Hong Kong15,16 and the prevalence of asthma in 13- and 14-year-old Hong Kong children was reported to be 12% in 1996.15 Many asthma patients believe that certain food can trigger exacerbation of asthmatic symptoms. Available data A recent survey of patients attending an asthma and allergy clinic in Melbourne revealed that 73% of patients attributed exacerbation of asthma to specific food intake and 61% of patients modified their diet as a result.17 In another community survey,18 21% of asthmatic subjects claimed to have food-induced exacerbation. However, controlled studies revealed that wheeze associated with other symptoms, or as the only manifestation of food allergy, did not confirm such high prevalence. Bock and Atkins reported that 68% of 410 asthmatic children gave a history of food-induced wheezing although this association was confirmed by double-blind, placebocontrolled food challenges (DBPCFC) in only 24% of cases.19 Oehling and Baena also reported that only 8.5% of patients had food-induced bronchospasm among 284 asthmatic children.20 The prevalence was highest among children with atopic dermatitis and food allergy. Food-induced asthma should be suspected in the following situations:
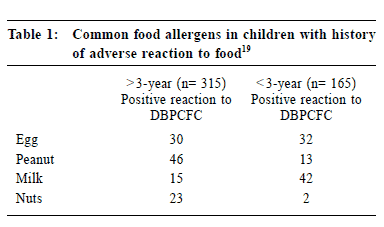
Milk and egg are the commonest food allergens identified in children less than 3 years old, whilst peanut and egg are the commonest food allergens in children older than 3 years of age (Table 1).19 Patients who have asthmatic symptoms associated with eating processed potatoes, shrimp, or dried fruit or with drinking beer or wine should be suspected to have sulfite sensitivity.21 Two recent studies22,23 were performed to screen asthmatic patients for food-induced asthma attack. The authors concluded that food allergy was a rare trigger for asthmatic symptoms, usually occurring in younger asthmatic patients, 12.5-year vs 16.2-year (p<0.01). James et al 24 studied the effect of various types of food on asthmatic patients, and reported that food-induced allergic reactions were highly variable. These reactions do not necessarily include respiratory symptoms or be associated with increased airway hyper-responsiveness. In another study,25 approximately 50% of the children with a history of food-induced asthma had an increase in bronchial hyper-reactivity following blinded food challenges. Bronchial hyper-responsiveness in such cases was observed usually 90 minutes after challenge and tended to subside by 150 minutes.
Clinical features In general, food-induced asthma occurs within minutes to 1 hour following food ingestion. Patients may develop pruritus, epiphora, rhinorrhoea or itch in the mouth. This could progress to deep, repetitive coughing, shortness of breath, and wheezing. Acute attacks of asthma could be severe and could progress to anaphylaxis or even death within 2 hours of challenge. However, one must also be aware of the late asthmatic response, i.e. peak change that occurs 4 to 6 hours after challenge. This late response to food challenge has also been reported.22,23 Wheeze as the sole symptom of food allergy rarely occurs.23,26 Responses to food such as peanut or fish usually persist from childhood into mid and late adult life, while reaction to eggs, milk, soy and wheat are more likely to remit after a few years. Peanut is the major food allergen identified in USA and Europe.27 It represented 28% of food allergies and occurred under 1 year of age in 46% of cases in one series.28 The most common symptom is atopic dermatitis (43%), followed by hoarseness (35%), asthma attack (14%), anaphylaxis (6%), gastrointestinal symptoms (2%) and oral syndrome (0.7%).27 It has been suggested that young atopic children should avoid peanuts containing food to prevent the development of peanut allergy.29 Clinical efficacy had been reported from exclusion diet,22 in genuine cases of food-induced asthma. Food-induced asthma is confirmed if double blind placebo control food challenge (DBPCFC) induces wheeze, a significant drop in FEV1, or a positive methacholine challenge (PD20FEV1) on the test day but not on the placebo day.30,31 Following identification of the allergenic food, strict elimination of that food is the only treatment proven effective to prevent reaction. If a patient accidentally eats a food product to which he or she is sensitive to and develops life-threatening anaphylaxis or severe asthma symptoms, he/she should be treated immediately with subcutaneous adrenaline, inhaled bronchodilators and systemic steroid32 accordingly. Diagnosis of food-induced asthma Food-induced asthma should be suggested if a patient or his or her carer volunteers a close association between certain food and asthma symptoms. Confirmation of this suspicion is difficult as skin tests could be unreliable.22 Serum-specific IgE is limited by availability of foodspecific IgE as well as poor correlation with asthma occurrence. DBPCFC remains the gold standard for diagnosis of food-induced asthma and referral to centres with this expertise is advised.18 Conclusion In summary, genuine food allergy may provoke respiratory symptoms in around 10 to 20% of patients with asthma. It is often associated with other extrapulmonary symptoms, e.g. urticaria. This contrasts with the much higher perceived prevalence of food-induced asthma exacerbation by the general public. Nonetheless, it is important to recognise the presence of food induced asthma in individual patients as specific avoidance measure could be adopted. Key messages
D K K Ng, MBBS, MMedSc, FRCP, FHKAM(Paed) K B Tang, Correspondence to : Dr D K K Ng, Consultant Paediatrician, Department of Paediatrics, Kwong Wah Hospital, Kowloon, Hong Kong. References
|
|