April 2002, Vol 24, No. 4 |
Update Articles
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stroke and heart disease prevention through lipid management*R S Scott HK Pract 2002;24:179-184 Summary Coronary Artery Disease (CAD) and stroke are more prevalent in patients expressing the 'Atherogenic Lipid Profile' viz: high triglyceride concentrations and low levels of High Density Lipoproteins (HDL) with accumulation of small dense Low Density Lipoproteins (LDL). The most common clinical examples of patients with these unfavourable lipid profiles are obesity particularly android fatness, insulin resistance, type 2 diabetes and familial combined dyslipidaemia. World wide, many populations, including those in the Asian region are showing rising rates of obesity and diabetes. The recently published NCEP guidelines emphasise the cardiovascular risk of obesity, insulin resistance and diabetes. 摘要 有動脈瘤血脂成份,即甘油三脂過高、高密度血脂蛋白過低及低密度血脂蛋白過高的病人較易患冠心病和中風。常見於肥胖(尤其是男性型肥胖)、胰島素阻力、第二型糖尿病和家族性異常血脂症的病人。全世界包括亞洲肥胖症和糖尿病的比率日漸上升。NCEP最近發表的指引強調肥胖、胰島素阻力和糖尿病是最重要的危險因素。 Effective management to reduce coronary event rates entails assessment of all risk factors. Treatment of risk factors that are even within population range remains worthwhile in high-risk subjects, since there is a continuous risk gradient from lower to higher values (e.g. Homocysteine, LDL-C). Such approaches may be especially useful in reducing CVD risk in Insulin resistant and diabetic subjects who frequently present with a cluster of risk factors. Specifically for lipid management, it is not enough to lower LDL-C levels alone. HDL and triglyceride rich lipoproteins need also to be modified if cardiovascular risk is to be reduced. This does not in any way reduce the importance of lowering LDL-C as there are several studies that have included patients with the atherogenic lipid profile, including those with diabetes mellitus, in which reduced CVD event rates were observed after LDL-C reduction (Heart Protection Study (HPS), CARE and LIPID studies). The clinician will need to use diet, exercise, nutritional agents, and specific drugs, combining their benefits and exploiting their individual medical actions to minimise CVD risk through correction of the atherogenic lipid profile in these high risk patient groups. Stroke, ischaemic heart disease and other vascular syndromes are major causes of morbidity and mortality in all developed and developing countries. In Asia, these problems are prevalent1,2 and with the epidemic expression of the Insulin Resistant Syndrome, will over the next decade become as prevalent or even more so, than in European and US communities. Individuals with insulin resistance, obesity, diabetes mellitus, impaired glucose tolerance, a smoking history and hypertension are more prone to such clinical events. Effective clinical intervention requires regulation of all risk factors such as dietary disorganisation, excess weight, lack of exercise, blood pressure, smoking, diabetes control and dysfunctional lipoprotein metabolism. With particular reference to lipid modification and prevention of stroke and heart disease there are a number of questions that, despite the reports of the major intervention trials, are inadequately answered. These are:-
Cholesterol and LDL-C treatment targets There is strong advocacy for treating LDL-C and cholesterol levels to low values in high-risk patients. The newly released ATPIII, NCEP guidelines3 state that LDL-C levels should be under 100mg/dL (2.6mmol/L). The major trials that support aggressive LDL-C reduction include the Post Coronary Artery Bypass Grafting trial4 (Post CABG), the AVERT trial,5 extrapolated data from 4S,6 and most importantly the recently presented HPS.7 In the Post-CABG trial, the objective was to compare composite clinical end points of death from CVD or non-fatal MI/CVA or CABG or angioplasty across two groups. The first group was treated aggressively with the objective of achieving LDL-C levels between 60-85mg/L (1.6-2.2 mmol/l). The less aggressive treatment group aimed for LDL-C levels between 130-140mg/dL (3.4-3.6 mmol/l). After 7.5 years the intensively treated group had a 30% reduction in re-vascularisation procedures and a 24% decrease in composite end points compared to the less aggressively treated group. Patients with ischaemic heart disease were studied in the AVERT trial. The two treatments compared were Atorvastatin 80mg/day against angioplasty and usual care, which could include lipid-modifying therapy. The achieved LDL-C cholesterol levels in the two treatment groups were 77mg/dl (2.0mmol/L) in the Atorvastatin treated group versus 119mg/dl (3.1mmol/L) in the angioplasty usual care group. By the end of the eighteen month treatment period, there were significant reductions in time to the next ischaemic event (P=0.027) in the Atorvastatin treated group. In the HPS,7 no threshold effect for benefit based on achieved LDL-C was observed. The mean level of LDL-C on treatment was 2.4mmol/l and there was a similar reduction in risk for vascular events amongst patients treated to below 2.0mmol/l as against those achieving higher LDL levels on treatment. On the basis of these trials there would seem to be substantial evidence to recommend that high-risk patients achieve LDL-C levels of 2.0mmol/l or less. The patient with established heart disease who already has quite low LDL-C levels (3mmol/L or less) however poses a different question. The combined data set from CARE and LIPID was not supportive for a powerful reduction in recurrent cardiac events amongst subjects with such untreated LDL-C levels,8 and these values are more typical of diabetic and insulin resistant patients presenting with ischaemic heart disease than those with marked LDL-C elevations. Many patients who have LDL-C levels in the broad range of 2 to 4mmol/l are dyslipidaemic, showing raised triglyceride levels and low HDL levels. The HPS7 does fortunately appear to answer this issue as there was a 24% risk reduction for vascular events amongst patients with baseline levels even less than currently recommended by ATPIII, NCEP guidelines.3 The first line agents for reducing LDL-C levels are the HMG CoA Reductase Inhibitors. There is an order of potency in these agents with Rosuvastatin being more powerful than Atorvastatin and in turn Atorvastatin being more powerful than Simvastatin.9 Pravastatin, Fluvastatin and Lovastatin have low potency based on mg dose versus % LDL reduction. Amongst patients with a cholesterol level of 5.5mmol/L and a LDL-C of 4mmol/L, a dose of 20mg of Atorvastatin would result usually in a reduction of LDL-C to target values of 2.3mmol/L or less in at least 50% of such subjects. The corresponding dose of Simvastatin that would achieve these target LDL-C levels would be 40mg and for Pravastatin 80mg/day. For patients with higher LDL-C levels (>5.0mmol/l), a combination of a second lipid modifying agent together with the maximum dose of a Statin (80mg/day) would be often required if these chosen LDL-C target levels were to be achieved (Table 1). Drugs that may be combined with Statins to further reduce LDL-C by 0.5 to 1.0mmol/l are the Bile Acid Sequestrants, Nicotinic Acid and Fibrates. Other agents acting on cholesterol absorption are viscous fibres or plant sterols supplied in the form of margarines and newer agents such as Ezetimibe (Merck USA). Other agents being exploited for their lipid modifying effects and which will be available in the near future include the ag agonists for the nuclear receptor PPAR.
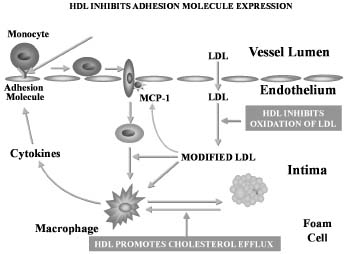
The problems with high dose Statins and with combination therapies that include a Statin are increased risk of side effects particularly muscle aches and the rarer complication of rhabdomyolysis. Changes in liver tests also are more frequent. Polypharmacy is inevitably associated with compliance issues and still unresolved is whether there are long term adverse effects of treating to extremely low LDL-C levels. Finally amongst patients who are primarily dyslipidaemic (that is, population average LDL-C, high triglyceride and low HDL levels), LDL-C may not be the only target. Approximately 50% of patients with coronary artery disease, European or Asian,10 show an Atherogenic Profile of low HDL, high triglycerides and unremarkable elevations of LDL-C. Lipoprotein fractions other than LDL may be as, or more important for prevention and delay of CVD events. Cardiovascular risk status in individuals with the 'Atherogenic Lipid Profile', may not necessarily be optimised by treatment with HMG CoA Reductase Inhibitors alone. Non-LDL treatment targets HDL is pivotal for inhibition of atherosclerosis (Figure 1). It has a variety of functions such as inhibition of adhesion molecules, inhibition of LDL oxidation and reversal of cholesterol transport mechanisms. Obesity, diabetes mellitus, impaired glucose tolerance, stroke, vascular disease, polycystic ovarian syndrome, and sleep apnoea are common conditions that are often associated with low HDL levels. Many patients with insulin resistance have low HDL levels. These patients often have android obesity and may have cutaneous markers such as skin thickness, skin tags or peduncles, pigmentation over bony prominences, acanthosis nigricans. Insulin resistant patients are prone to cardiovascular disease,11 diabetes mellitus, gout, infertility, reduced libido, hypertension, pulmonary hypertension, and heart failure (Table 2). Their lipoprotein profiles are characteristically of moderately elevated triglycerides with low HDL's and small dense LDL and small dense HDL. It is the composition of the lipoproteins that confers their marked atherogenicity.
Strategies for modifying HDL and lipoprotein function in insulin resistance need to focus on exercise, weight loss and dietary change avoiding refined carbohydrate and saturated fat intake, increased mono-unsaturated fats, and omega 3 fish oils. Medications that are favourable for an effect on HDL are the Fibrates, the Glitazones, ag mixed PPAR agonists, Metformin and Statins. The PPARa activators (fibrate agents) have important anti-atherogenic effects by increasing HDL and apo A1 and apo A2 and decreasing VLDL and triglycerides. They also have anti-inflammatory actions and promote fatty acid catabolism. The importance of fibrates for cardiovascular prevention is reflected in the VA-HIT trial,12 which used Gemfibrozil. Despite no change in LDL-C levels from baseline (2.9mmol/L), there was a decrease in rates of non-fatal myocardial infarction or death from CAD by 22%. Improvement in event rates was in part a function of change in HDL concentrations, but this did not explain outcomes completely. An action of PPARa activators on non-lipid parameters (pleiotropic actions) may be of importance. The described anti-inflammatory properties, the removal of cholesterol from macrophages and anti-thrombotic effects may be contributory factors. Whereas Statins do increase HDL, the effect is often small and it is worth noting that all are not equal for this effect. At higher doses, Atorvastatin appears to lose some of its HDL elevating effects. This suppressive effect is not seen with Simvastatin or Rosuvastatin. PPARg activators (Glitazone agents) have also been found to have an effect on HDL and LDL composition. They also improve insulin sensitivity and reduce glucose levels, and are described as having anti-inflammatory effects on the vessel wall suggesting a potential role in preventing atherogenesis. To date, these agents remain unreported for cardiovascular benefit in trials. The targets for non-LDL lipoproteins are HDL greater than 1mmol/L in males and 1.2mmol/L in females, and for triglycerides, less than 1.5mmol/l. Ideally these targets should be accompanied by a reduction in apoB containing lipoproteins (e.g. LDL-C) so that the apoB level is not greater than 0.9g/l. Lipoprotein-endothelial interactions Agents that stabilise the endothelium and reduce endothelial inflammation, although not directly influencing lipoproteins are likely to be anti-atherogenic. Fibrates, PPARg agonists and ag mixed PPAR activators appear to exert anti-inflammatory effects that may be independent of any action on lipids (pleiotropic effects). Fish oil supplementation, homocysteine reducing therapy with Folic Acid, flavonoids, anti-oxidant agents and oestrogens all affect endothelial function and may be potentially anti-atherogenic. It is thus interesting to observe that in the major lipid modifying trials (Table 3) of HPS, 4S, CARE, LIPID and VA-HIT using Statins and Fibrates, there have been reductions in stroke event,13,14 rates. In the 4 Statin trials the risk reductions ranged between 19-31% and in the VA HIT trial using Gemfibrozil, the risk reduction was 25%. It is difficult to explain these changes in stroke event rates entirely on reductions to LDL-C or change in other lipoproteins. An effect, either indirectly or directly on the vessel wall and the endothelium seems plausible.
Improving clinical practice In spite of lipid modifying clinical trials being so positive for reduced stroke and heart event rates, there have been a number of studies showing that clinicians have been slow to apply this information cohesively and effectively. Reports suggest that up to 50% of patients with established vascular disease and high-risk status are untreated. Even if treated, there may be low compliance both short and long term, inadequate monitoring and with too few reaching LDL-C and other lipid targets that are currently advocated.15,16 Clinicians need to adopt strategies appropriate to their environment to ensure that patients who are at high risk are identified and treated effectively. Conclusion Amongst patients with high cardiovascular risk, LDL-C reduction is important for reducing recurrent events. It may, however, not always be possible to achieve the very low levels of LDL-C being promoted currently, let alone the more conservative LDL-C targets of <2.6mmol/l of the NCEP. High dose of Statin (80mg/day) and combination drug treatments may be needed. LDL-C is not the only therapeutic lipoprotein target. Low levels of HDL need assertive correction and this implies decrease of triglyceride rich lipoproteins, achieving fasting triglyceride levels below 1.5mmol/l. Diet change, exercise and incorporation of special foods may be neglected in the belief that they are less contributory to risk reduction than medication. Non-pharmacological approaches are needed for reversal of the Atherogenic Lipid Profile. Careful use of drug combinations, exploiting individual and specific therapeutic actions, is an essential approach to effective cardiovascular disease risk reduction. The entire range of lipid-modifying agents, various metabolic agents such as the PPAR activators, commonly used cardiovascular drugs such as ACE Inhibitors, and nutritional substances appear to possess additional specific actions that can reverse endothelial dysfunction. However, no intervention program will reduce event rates if not continued, if used with poor compliance or if not achieving therapeutic targets. Key messages
* This article is based on the presentation 'Long Term Survival from Cardiovascular Disease: Powerful Strategies for Stroke and Heart Disease Prevention, Regulation of Lipoproteins' delivered at the Joint Scientific Meeting of the Hong Kong Colleges of Physicians, Paediatricians, Family Physicians and Pathologists 27-28 October, 2001. R S Scott, MBChB, BMedSci (Otago, NZ), PhD (Monash, Aust), FRACPC Correspondence to : Professor R S Scott, Director, Lipid and Diabetes Research, Christchurch Hospital, Christchurch, New Zealand. References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||