|
April 2003, Volume 25, No. 4
|
Update Article
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
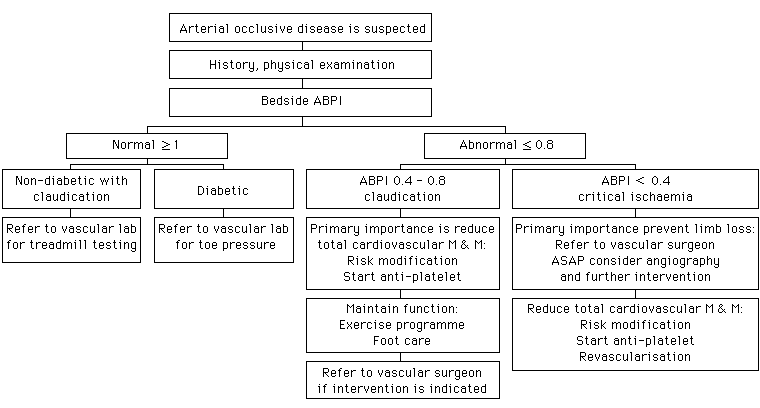
Management of chronic lower limb ischaemiaA C W Mui 梅中和 HK Pract 2003;25:165-175 Summary This article provides a general and broad review of the current management of arterial occlusive disease of the lower limb. Chronic lower limb ischaemia presents with different degrees of severity ranging from asymptomatic at one end of the spectrum to critical ischaemia at the other with intermittent claudication in between. Important established facts of the disease are reiterated. Rationale for the management and the aggressiveness of treatment of the different severities are discussed. 摘要 本文對下肢動脈閉塞性疾病的新近治理方法作出廣泛討論。下肢周圍血管病的臨床表現因缺血程度不同而異。 從無症狀至功能性缺血其表現為間歇性跛行,遞增至臨界性缺血即靜息痛或組織壞死。本文重申已確定的理據, 並陳述治理的基本原則及針對病程的治療方案。 Introduction Prevalence of atherosclerosis increases with age and the elderly represent the fastest growing section of our population. In our community atherosclerotic arterial disease and its complications are becoming increasingly common. It has been shown that pattern and distribution of arterial lesions that produce critical lower limb ischaemia in Chinese patients are not "small vessel disease", and most of them will benefit from reconstructive surgery.1 In fact there is no obvious difference between Chinese and other racial groups.2 Atherosclerosis is a systemic disorder. Patients with lower extremity ischaemia have a significantly increased risk of stroke, myocardial infarction and cardiovascular death. At least 10% of patients with lower limb ischaemia have cerebrovascular disease and 30% have coronary heart disease.3 Cardiac and cerebrovascular complications account for 75% of the eventual mortality in this patient population.4 About 40% of patients with coronary heart disease or cerebral circulatory disease will also have arterial occlusive disease of the lower limb.5 The importance of identifying patients with lower extremity arterial disease extends beyond its impact on the lower limb. Lower limb ischaemia should be viewed as a sign of diffuse and significant arterial disease.6 They are candidates for aggressive risk factor modification and anti-platelet therapy that can slow down disease progression in the lower limb and reduce the risk of subsequent cardiovascular event. As arterial occlusive disease of the lower limb presents in different severity, asymptomatic at one end and critical ischaemia at the other with intermittent claudication in between, treatments differ accordingly
Asymptomatic occlusive arterial disease of the lower limb Asymptomatic occlusive arterial disease is usually detected as an incidental finding during investigation of disease of other vascular bed. Absent distal pulses are easily confirmed by physical examination. Hand-held Doppler flow-metre can be used to measure the Ankle Brachial Pressure Index (ABPI). The device is simple to use and is inexpensive. The index is determined by comparing the systolic blood pressure measured at the ankle with that of the brachial artery. This provides reproducible and objective documentation of significant arterial occlusive disease and can be used as baseline to assess disease progression (Table 1).
Lower extremity arterial disease is generally defined by a resting ankle-brachial pressure index of less than 0.9. It is associated with a >50% diameter stenosis of the artery. When an ABPI <0.9 is used as a reference standard, the prevalence of asymptomatic arterial disease of the lower limb in the 55- to 74-year-old age group is about 10% in the West.7 There is no comparable local data. A word of caution is that possible error may arise from advanced arterial calcification. This situation is particularly common in diabetic patients and patients with chronic renal failure. Thus the sensitivity of ABPI in these groups of patient is lower. This can be overcome by toe pressure measurement as digital arteries are not involved by this calcification process. Management
The principal objective is to reduce cardiovascular mortality and morbidity. Aggressive risk factors identification and modification through patient education is an important part of the treatment. The main risk factors for atherosclerosis are cigarette smoking, diabetes mellitus, hypertension, hyperlipidaemia and hyperhomocysteinemia. Smoking and diabetes are the most important and most prevalent.
All patients with occlusive arterial disease should be strongly advised to stop smoking. Stopping smoking slows the progression of the disease and reduces the risks of myocardial infarction and death from vascular cause.7 Major amputation is more common among claudicants who are heavy smokers, and those who continue to smoke. Good control of blood glucose levels delays the onset of microvascular disease.8,9 Patients with diabetes should have a tight control of blood sugar level. Fasting blood glucose should range from 80 to 120mg/dL, postprandial level at <180mg/dL; and haemoglobin A1c should be <7%. Intermittent claudication Claudication is from Latin which means "to limp". Exercise increases the demand for muscle blood flow but in an atherosclerotic vascular bed the supply cannot increase sufficiently to meet the demand. The symptoms of intermittent claudication can be differentiated from other causes of exercise-induced pain (Table 2). Claudication is characterised by reproducible exercise-induced muscle pain that is relieved by a brief period of rest. There should be little day-to-day variability in its severity as well as the walking distance that precipitates the pain. Depending on the anatomy of the arterial lesions, the patient may present with buttock, thigh or calf claudication.
Prognosis of the affected limb of patients with intermittent claudication is relatively benign but their life prognosis is compromised. Only about a quarter of patients with intermittent claudication will significantly deteriorate over time. Surgical intervention is required in a fairly consistent 6% of claudicants during long-term follow up.11,12 Annually approximately 1% claudicants will require major amputation.13 Compared with age and sex-matched controls, they have a six-fold increase in cardiovascular mortality.14 Thus the real threat for the patient with intermittent claudication is not as much from potential limb-loss as from premature cardiovascular mortality. The mortality is approximately 30% at 5 years, and 50% at 10 years. In fact, the prognosis is comparable to that following resection of a Duke's B carcinoma of the colon. Physical examination Physical examination should cover the whole cardiovascular system and not just the lower limbs. Trophic changes of the involved limb are common. Loss of distal pulses at different levels may allow the prediction of the location of the arterial lesion. However, occasionally collateral circulation around a single level stenosis can produce pulses in the distal arteries of a claudicant. Investigations Basic haematological and biochemical studies should include a complete blood picture, renal function test, blood glucose level and lipid studies. These help to identify the associated risk factors and concurrent diseases.
Treadmill exercise test is helpful when the clinical diagnosis of arterial claudication
is not clear-cut. It is useful in patients whose history and physical finding do
not match and in patients with mixed pathology. The ABPI is measured before and
after a patient walks at a standard speed and gradient until claudication pain is
experienced, or a set time-limit has been reached. There should be a drop in the
ABPI of
Segmental pressure and the ABPI can also quantify the severity of disease. These can be used to select surgical candidate for arteriogram and percutaneous intervention during the same angiographic session. The spatial resolution of MRA (magnetic resonance angiography) does not yet match that of conventional angiography, and the technique is prone to exaggerate the severity of pathology.16 With advances in new technology and expertise, MRA may eventually prove to be an effective non-invasive and the preferred vascular imaging technology. At this moment, digital subtraction arteriogram is still the gold-standard. It is an invasive investigation with a morbidity risk of about 1% and mortality risk of 0.16%.15 It is expensive and an unpleasant experience for the patient. In intermittent claudication, angiography is indicated only when a decision has been made to intervene. Management Risk factors modification, regular walking exercise, meticulous foot-care, and drug therapy will benefit patients with intermittent claudication. However, despite such conservative treatments, if symptoms deteriorate and become unacceptably limiting, then interventions such as angioplasty or surgery are indicated.
Regular walking exercise can significantly increase walking distance so that intervention becomes unnecessary. This will also alleviate anxiety, relieve unnecessary self-imposed restrictions with improved quality of life when patients understand that the exercise-induced pain is not harmful and indeed walking exercise can gradually increase the pain-free walking distance. However, these patients should avoid strenuous exercise. Patients with unstable angina pectoris, or symptomatic congestive heart failure should be excluded from exercise therapy. Regularity rather than intensity should be the hallmark of all exercise programmes. Exercise therapies range from recommending unsupervised walking within the community to a formal supervised exercise programme on a treadmill. The latter is effective but expensive. Due to limited resources, our usual practice is to instruct patients to walk until claudication occurs, then rest until the pain abates. The cycle should be repeated for about an hour a day and at least three times a week. To improve compliance, the rationale of the exercise programme should also be explained. Available literature suggests that exercise therapy is the most consistently effective treatment for intermittent claudication. A meta-analysis of these studies showed an average of 179% increase in initial claudication distance and a 122% increase in maximal walking distance on the treadmill.17 Exercise therapy also has the additional benefit of favourably modifying other cardiovascular risk factors. The biggest deficiency of exercise therapy is poor patient compliance. Professional advice on foot-care should be offered to patients. Foot wear and foot protection are important to prevent minor trauma. Patients should be careful with nail or callous trimming. They should walk with appropriate foot wear and avoid exposing their feet to strong chemicals, disinfectants, extreme heat or cold. Feet should be kept clean and dry. Patients are encouraged to inspect their feet daily for abrasion, ulceration or infection which should be reported to their physician immediately. Clinicians should never rely on symptoms alone to identify foot ulceration. Diabetic neuropathy and retinopathy may deprive the ability of a diabetic patient to experience pain or to detect a skin abrasion before it is too late. Careful examination of the feet including interdigital spaces should be an integral part of a medical consultation. Patients with intermittent claudication often receive drug treatment for other coexisting disease, such as hypertension and diabetes. Some also have anti-lipid medication for risk factors modification, and an antiplatelet agent as prophylaxis against thrombotic events associated with atherosclerosis. Vasoactive agents with different mechanisms of action may have an adjunctive role in a subgroup of patients.
Aspirin therapy significantly reduces risks of non-fatal myocardial infarction, non-fatal stroke and death from all vascular causes. After meta-analysis of studies of antiplatelet therapy, the Anti-platelet Trialists' Collaboration concluded that aspirin reduced the risk of fatal or non-fatal cardiovascular events from 11.9% to 9.5%.18 Aspirin is recommended for secondary prevention in patents with cardiovascular disease, including lower limb arterial occlusive disease.19 Antiplatelet therapy also helps to maintain graft patency following bypass surgery.20,21 It is worthwhile to note that low-dose aspirin was as effective as high-dose aspirin.22 Clopidogrel, a thienopyridine derivative, was shown to be significantly more effective than aspirin in the prevention of vascular events.23 Our usual regimen is to prescribe a daily dose of 100mg enteric-coated aspirin for patients with peripheral vascular disease. Clopidogrel, an expensive alternative, is reserved only for patients for whom aspirin is contraindicated. Clinical trials with pentoxifylline or naftidrofuryl have shown statistically significant improvement in walking distance; however the clinical benefit was small. Cilostazol, a phosphodiesterase type 3 inhibitor, inhibits platelet aggregation, suppresses the formation of arterial thrombi and vascular smooth muscle proliferation. It also causes vasodilatation.24-26 At least 4 randomised placebo-controlled trials have shown that cilostazol improved both pain-free and maximal treadmill walking distance in patients with intermittent claudication.27-30 Our practice is to prescribe a short course of this drug for severe claudicants and continue its use if sufficient benefit is observed. Recently a prostacyclin analogue with antiplatelet and vasodilating properties, oral beraprost sodium, was shown to have modest benefit in improving walking distance.31 Other medications such as the chelating agents and vitamin E have no proven value on claudication. There is no evidence that lipid-lowering agents will alter the course of lower limb ischaemia. However, three major secondary prevention statin trials, LIPID,32 CARE33 and the 4S,34 have demonstrated remarkable vascular benefits in patients who have presented with acute coronary syndromes. As lower extremity ischaemia is a sign of diffuse atherosclerosis, the current recommendation is to achieve a serum LDL cholesterol concentration of less than 100mg per deciliter or 2.6mmol per litre and a serum triglycerides concentration of less than 150mg per decilitre or 1.7mmol per litre. As most patients will have sufficient improvement with conservative management, the risk of both early and late complications of invasive procedures such as angioplasty or bypass operations are usually not justified. The decision for intervention is arrived at by the patient after balancing the degree of disability against the procedural risk and durability of the planned procedure. Disability is assessed in terms of the claudication distance and the functional demands or job requirement of the patient. Concurrent disease that would limit exercise, even if claudication improved, such as angina or chronic respiratory disease should also be given appropriate consideration. Although percutaneous endovascular procedure is less invasive than surgery, in general it does have a complication rate of about 3%. Furthermore, after investigation, only about 10% of claudicants are found suitable for conventional angioplasty. The durability of angioplasty of infrainguinal arteries is sub-optimal35 and may not be more effective than programmed exercise in the long-term. However, balloon angioplasty is still the preferred option if a suitable lesion (e.g. short stenosis) is found. Surgical reconstruction of the aortoiliac and above knee femoropopliteal segment has been performed with excellent results. However, infragenicular bypasses are associated with less favourable long-term results and higher operative morbidity compared to above knee bypasses. Below knee bypass is not recommended for intermittent claudication. The crucial issue is in patient selection for maximum and lasting benefit from surgical reconstruction. Critical lower limb ischaemia In terms of both limb survival and patient survival, critical leg ischaemia carries a far worse prognosis than claudication. There is an annual mortality of 25%.36 It has been repeatedly shown that at least 20% of patients were dead at one year.14,37,38 Most (up to 95%) of patients who present with gangrene, and 80% of those presenting with rest pain, are dead within 10 years. Critical limb ischaemia results from a reduction in perfusion to the extent that the basal metabolic needs of the tissues are not adequately met. This commonly leads to rest pain, non-healing ulcer, or ischaemic gangrene. This is termed "critical" because the risk of limb loss is significant and imminent in the absence of successful revascularisation. The leisurely investigation of these patients in a primary care setting is inappropriate. They should all be promptly referred to a vascular centre, with multi-disciplinary team approach to this often multi-system disease.3 History Many patients with critical lower limb ischaemia have history of claudication; but some patients are too sedentary to claudicate and present initially with critical ischaemia. Patients with diabetes have a widespread distribution of arterial disease, with more frequent involvement of the tibial arteries. In these patients, claudication may not be a prominent symptom. Ischaemic pain is always worst in the distal part of the foot where perfusion is poorest. Typical ischaemic rest pain is worse at night when the patient is resting horizontally. The pain is partially relieved by using gravity to improve the perfusion to the distal part of the foot. The classical pattern of sleeping with the foot dangling over the side of the bed is too characteristic to miss. Adequate pain control may require the use of strong analgesics. Physical examination Buerger's sign, cadaveric pallor on elevation and rubor on dependency, is an important sign of poor perfusion. Poor capillary filling indicates a propensity to ischaemic gangrene and ulceration. Other findings include: trophic changes, non-healing ulcer and gangrenous digits. A cold extremity and loss of foot pulses are the usual findings. Investigation Basic investigations are similar to those of intermittent claudication. Patients with critical lower limb ischaemia often have an ankle pressure of less than 50mmHg. The tibial vessels of patients with long standing diabetes or chronic renal failure are often severely calcified and are incompressible. The ABPI may be falsely elevated. As calcification rarely involves the digital vessels, toe systolic pressure is measured in these patients. Toe systolic pressure of less than 30mmHg is indicative of critical ischaemia, and cutaneous lesions are unlikely to heal without revascularisation. Angiogram to the level of the pedal arch provides a road map and is a prerequisite for revascularisation surgery. Management Apart from risk factors modification, antiplatelet therapy and foot-care, the ulcerated or gangrenous part also need meticulous wound care. Infection should be aggressively treated. Early intravenous antibiotic and wound debridement are indicated where there is spreading infection. Concurrent diseases require appropriate and prompt management before, during and after interventions so that risks are minimised. In view of the poor mobility achieved by the amputees in various studies,40 vascular reconstruction for critical limb ischaemia should be offered instead. Bypass surgery has a significant mortality and morbidity, but after successful bypass, the patient with an amputation-free survival has a much better quality of life. Bypass surgery is justified even in a suitable octogenarian if the alternative is major amputation.41,42 Ideally, patients should be treated with the least morbid but most successful and durable procedure, be it endovascular or surgical bypass. An endovascular procedure alone for critical lower limb ischaemia, which is usually associated with a diffuse and multi-level pattern of atherosclerosis, is often neither feasible nor durable. Occasionally, frail patients are best treated with less invasive interventions, though durability may be compromised. However, the temptation to pursue the minimally invasive procedure in the relatively healthy individual with multi-segment disease should be resisted. There is a failure rate associated with the dilatation of each segment of artery, and failure at any site could negatively affect the entire reconstruction. Surgical bypass of the multi-segment occlusions in the main trunk arteries is the mainstay of treatment of critical limb ischaemia.43 The decision should be based on what is best for the individual patient and not simply on technical feasibility. Distal bypass even to the foot arteries should be attempted unless there is no salvageable weight bearing area in the foot. Primary major amputation is appropriate in patients with tissue loss extending into the calcaneum, or in patients with severe fixed flexion-contractions and those who are wheelchair-bound. Advanced age alone, treated malignancy or contralateral amputation are not contraindications for vascular reconstruction. From time to time, a multi-disciplinary approach combining endovascular and open procedures may offer the best hope for limb salvage and improved quality of life. Occasionally, intravenous prostanoid to improve the microcirculation of ischaemic tissue is useful for the treatment of rest pain or small ulcers in patients with inoperable vessels.44,45 Buerger's disease Thromboangiitis obliterans is a non-atherosclerotic inflammatory disease affecting the small arteries and veins in the distal part of the upper and lower extremities. Although more common in Asia, it accounts for only a small proportion of patients who present with lower extremity tissue loss. In our experience this condition has often been over diagnosed in our locality. It should be diagnosed only in a smoker with no known diabetes. The age of onset of symptoms of ischaemia of the distal extremity has to be before the age of 45. Complete discontinuation of use of tobacco in any form is the only way to stop the disease process. Apart from wound care and conservative ablation of the ulcerated or gangrenous digits, other intervention is neither effective nor necessary. Final comment Compared with the Western countries, the prevalence of peripheral vascular disease in Chinese may be lower. However, the pattern and the results of treatments of arterial occlusive disease of the lower limb in Chinese patients are similar to those of their Western counterparts. This disease is only part of a systemic disease "atherosclerosis". In secondary prevention of cardiovascular morbidity and mortality: patient education, aggressive risk factors modification, and anti-platelet therapy should be the initial management of all patients including the asymptomatic patient, patient with intermittent claudication or one with critical lower limb ischaemia. An invasive procedure is not justified in the asymptomatic. In the non-limb-threatening situation of intermittent claudication, invasive investigation and procedures should be limited to those patients in whom there is an indication to intervene, namely those having unacceptably severe symptoms. However, to prevent limb loss, patients with critical limb ischaemia should undergo investigation and plan for revascularisation unless there are over-riding contraindications. Urgent referral to a vascular surgeon is of paramount importance to prevent limb loss in critical limb ischaemia. Key messages
L K M Chiu, MBBS, FRCS, FHKAM(Surgery)
Senior Medical Officer, Division of Vascular Surgery. A K AhChong, MBChB, FRCS, FHKAM(Surgery) Consultant, Department of Surgery, Kwong Wah Hospital. Correspondence to : Dr L K M Chiu, Division of Vascular Surgery, Department of Surgery, Kwong Wah Hospital, Kowloon, Hong Kong.
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||