
|
March 2005, Volume 27, No. 3
|
Update Articles
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Chemical pathology case conference - heavy metalsMichael H M Chan 陳浩明, Robert C K Cheung 張志強, Albert Y W Chan 陳恩和, Eric C W Lam 林青雲, Tony W L Mak 麥永禮, Anthony C C Shek 石志忠, Sidney Tam 譚志輝, Christopher W K Lam 林偉基 HK Pract 2005;27:94-102 Summary The term "heavy metals" should have been reserved for those metallic elements with an atomic mass of 200 or above, such as mercury (200), thallium (204), lead (207) and bismuth (209). However, it would be clinically relevant to include some lighter metals such as aluminium (27), arsenic (75), cadmium (112), cobalt (59) exposure to which is clinically undesirable and constitutes a health hazard. Heavy metals are normally present in biological fluids and tissues at very low concentrations [<100mg/g or part per million (ppm)] that are often below the detection limit of early analytical methods, and therefore also called "trace metals". The latter term generally also includes "essential trace metals", which are (i) naturally available in our diet, (ii) present in the body at relatively constant concentrations, (iii) physiologically important, and (iv) pathogenic if deficient. "Trace elements" is perhaps a more appropriate term if reference is made to include both metals and non-metals such as iodine, bromine and silicon. In this case conference, the biochemical profiles for a series of clinical cases are used to illustrate the precautions in the patient and sample preparations for metal analysis, the proper interpretation of the laboratory results, as well as the principles of investigation in patients suspected of heavy metal intoxication. 摘要 重金屬通常指金屬元素原子量大於或等於200或以上的金屬,例如水銀、鉈、鉛和鉍。 然而,臨床上亦包括某些原子量較輕的元素,如鋁、砷、鎘和鈷,可以因為過度接觸而導致不良反應, 危害健康。正常情況下,體液和組織中的重金屬濃度非常低〔<100毫克/克或百萬分之一(ppm)〕, 早期分析方法難以測出,故稱為微量金屬。其中包括必需的微量金屬,是指(i)經飲食自然吸收, (ii)體內濃度相對穩定,(iii)生理上有重要性,(iv)如果缺乏會引致疾病。 微量元素涵蓋了金屬和非金屬元素(如碘、溴、矽),是更恰當的名稱。這次臨床會議, 展示了這類病例的一系列生化特點,說明了病人和樣品準備的預防措施,闡述了懷疑重金屬中毒者檢查結果分析的原則。 Introduction In recent years, there have been increasing number of locally reported cases of heavy metal poisoning due to worsening environmental pollution1 or contamination, as well as a heightened alertness of the lay public and medical practitioners by the repeated warnings from overseas and local health authorities.2,3 Heavy metal poisoning can be associated with accidental over-exposure from industrial, dietary, drug-related, or even cosmetics-related sources. Signs and symptoms of heavy metal poisoning are usually subtle and not well defined because the effect of heavy metal toxicity can vary from gastrointestinal upset to severe neurological damage. The mechanism of this wide range of toxicity is due to the covalent binding of heavy metals to sulphydryl groups at the active site of important enzymes in various organ systems causing loss of function.4 Due to the difficulty in clinical diagnosis, laboratory screening for heavy metals is commonly requested for the diagnostic work-up of patients suspected of poisoning. However, the screening of heavy metals using a correct sample type is also of prime importance to the subsequent clinical diagnosis and management. Hair is of no doubt a convenient sample that may reflect recent exposure. However, contamination of hair samples with air particles, dust, wave and colouring treatments is commonly encountered.5,6 Moreover, the measurement errors contributed by weighing the hair samples will be enormous if only a few roots are collected for analysis. There is a recent report of three local paediatric patients with a questionable diagnosis of heavy metal poisoning purely based on hair analysis whom unnecessary chelation therapy had been prescribed.7 In view of the above pre-analytical variables in hair analysis and the potential harmful effects of unnecessary chelation therapy, the Hong Kong College of Paediatricians has issued a position paper in which the use of hair as sample for heavy metal analysis is not recommended.8 Therefore, laboratory diagnosis of heavy metal poisoning should rely on properly collected blood and urine samples. Does high urinary arsenic concentration always indicate poisoning? Arsenic is a very toxic chemical in its trioxide form with an oral lethal dose of about 3mg/kg of body weight when taken acutely. Exposure to arsenic compounds is usually industrial as arsenic is used in the manufacture of glass, pigment, wood preservative, and semiconductors. Sometimes it is used in homicidal attempts. One of the Indonesian human-right activists was recently poisoned with arsenic during a flight to Netherlands via Singapore.9 Arsenic is also used therapeutically in both traditional Chinese medicine10 [in the form of realgar (雄黃, arsenic sulphide)] for the treatment of peptic ulcer, as an antidote for snake or scorpion bite via external application, and in western medicine for the treatment of acute promyelocytic leukaemia. Acute exposure can give symptoms of headache, nausea, and severe gastrointestinal upset accompanied with intense abdominal pain, vomiting, and diarrhoea. If left untreated, dehydration followed by oliguria and circulatory collapse with encephalopathy and death will supervene. Chronic exposure causes gastrointestinal discomfort, thick erythematous areas of skin and pruritic pinpoint dermatitis, alopecia, and peripheral neuropathy. Patients with chronic exposure have an increased risk of developing carcinoma of the skin and lungs. After initial exposure, arsenic quickly enters and leaves the systemic circulation. Therefore, exposure is best diagnosed and monitored by its urinary excretion rate if a timed urine collection is available or its urine concentration normalised to the creatinine concentration. Case 1 In a survey of local normal reference interval for spot urine arsenic concentration, the following results were obtained from 5 ostensibly healthy teachers in a secondary school:
All teachers denied previous exposure to arsenic compounds in their workplace, nor having taken any Chinese medicine recently. Interpretation Urine arsenic excretion was elevated in all teachers except one. They worked together in the same environment but lived separately. With a negative occupational history, it is unusual to find such high prevalence of arsenic over-exposure. The cause of elevated urinary arsenic concentration is likely to be dietary. Non-toxic organic compounds of arsenic (arsenobetaine and arsenocholine) are well absorbed in the gut and abundant in seafood, particularly fish and shellfish. After a seafood meal, urine output of arsenic may increase substantially to about 50 times above normal values for the first day before the organic arsenic is completely excreted in the second days without any toxic effects.4 The abnormal results in this instance have been caused by the recruitment personnel forgetting to remind volunteers of the pre-analytical precaution of abstaining from seafood for 5 days before urine collection.12 The urinalysis should therefore be repeated with proper dietary preparation. When this patient preparation is not practicable, such as for urgent investigation of a suspected case of acute poisoning, or forensic examination of a post mortem sample, speciation of inorganic and organic arsenic is available from specialist centres.13 My serum aluminium concentration is high, do I suffer from dementia? Aluminium is widely distributed in our environment. Food and beverages contain a small amount of aluminium so that our daily dietary intake is estimated at about 5mg/kg of body weight. In some developed countries, the problem of acid rain may increase the solution of aluminium from soil, thereby contaminating the underground drinking water. Fortunately, gastrointestinal absorption of this metal is less than 1% in healthy individuals. Any absorbed aluminium will be excreted in the urine except in the lung tissue, where the macrophages retain aluminium-containing particles by phagocytosis. Upon absorption aluminium is transported to every part of our body by transferrin in the systemic circulation. After crossing the blood-brain barrier, it acts as a neurotoxin. In the 1970s, patients with renal failure accumulated aluminium through dialysis therapy. Aluminium contaminated dialysis fluid was found to cause this dialysis encephalopathy.14 Exposure of aluminium is best diagnosed by measuring its concentration in serum or blood since it is equally distributed in plasma and erythrocytes. Case 2 Aluminium was requested as a part of the investigation of a 55-year old woman who had normal renal function complaining of increasing forgetfulness for 3 months.
Serum Aluminium 10.8
0.4 in subjects with normal renal function Interpretation No pre-analytical precautions on blood sampling procedures had been given to the attending clinician by the laboratory. Consequently, an uncertified clotted blood bottle was used for the collection of this blood sample. In a specialist trace element laboratory, proper blood collection precautions and acid-washed specimen bottles are issued to ensure the quality of the blood sample, as environmental contamination of aluminium using non-acid-washed specimen bottles is very common. This patient was found to suffer from hypothyroidism documented with elevation of serum thyroid stimulating hormone (TSH) to 18.0 mIU/L (normal 0.3-4.2). A repeat blood sample collected in an acid-wash clotted blood tube showed a normal aluminium concentration. Heavy metal poisoning presenting with chronic abdominal pain Abdominal pain is a commonly encountered presenting symptom in general practice. Common causes of acute abdominal pain can be attributed to infective, surgical, and gynaecological or obstetrical aetiologies, while chronic abdominal pain may be caused by constipation, peptic ulcer, inflammatory bowel disease, and psychological complications of organic diseases.15 Although tactful history and thorough physical examination can assist clinicians to narrow down these differential diagnoses, laboratory investigations are often required to confirm the initial clinical suspicion. Case 3 A 31-year old Caucasian businessman complained of feeling dizzy on exertion, nausea, poor appetite, low abdominal pain, generalised muscle and joint pain, and constipation over a month. He was admitted to hospital for investigation. Physical examination showed pallor and low abdominal tenderness. His haemoglobin concentration was 8.6g/dL (normal 11.0-14.2) with markedly elevated reticulocyte count of 5.9% (normal <1.0) and mild basophilic stippling. Serum liver function test showed elevated total bilirubin concentration of 38mmol/L (normal <15) and alanine aminotransferase activity at 119 IU/L (normal <58). Hepatitis A, B, and C serologies were all negative. Serum haptoglobin concentration, lactate dehydrogenase activity, and direct and indirect Coombs' tests were also normal. As the patient is a Caucasian, acute porphyria was suspected. Urine and stool were collected for total porphyrins, d-aminolaevulinic acid (d-ALA) and porphobilinogen (PBG) studies during the time of abdominal pain reported:
Does he suffer from acute porphyria? Interpretation This patient was suffering from lead poisoning instead of acute porphyria. Lead, like other toxic heavy metals, binds to sulphydryl group of protein molecules causing functional inactivation. In the haem biosynthetic pathway (Figure 1), lead inhibits the activities of d-ALA dehydratase, coproporphyrinogen decarboxylase, and ferrochelatase causing an increase in d-ALA (accounting for increase in its urine concentration), copropor-phyrinogen III, and protoporphyrin IX concentrations (accounting for increase in urine and stool total porphyrins). Decreased haem biosynthesis eventually results in decreased blood haemoglobin concentration. Feedback inhibition of haemoglobin on d-ALA synthetase is thus removed causing more d-ALA to be synthesised. Inhibition of d-ALA dehydratase is one of the earliest sign of lead poisoning occurring at blood lead concentration of 0.5mmol/L. Although the clinical signs and symptoms of acute porphyrias are similar to those found in lead poisoning including abdominal pain, constipation, and peripheral motor neuropathy, they can be distinguished biochemically. Urine PBG is not significantly elevated in lead poisoning but increased in all types of acute porphyrias including acute intermittent porphyria (AIP), porphyria variegata (PV), and hereditary coproporphyria (HC). Urine d-ALA (a neurotoxin) is elevated in both lead poisoning and acute porphyrias limiting its usefulness in the biochemical investigation. Urine total porphyrins are increased in lead poisoning and hepatic porphyrias, as well as other diseases including erythropoietic porphyrias, infectious hepatitis, cirrhosis, haemochromatosis, acute and chronic alcoholism, haemolytic, aplastic and pernicious anaemias. The daily intake of lead is about 1mmol via drinking water and 1mmol from food. Once absorbed, lead rapidly accumulates in erythrocytes (95 %) with a half-life of 35 days before it is passed into the urine or is transferred to soft tissues including hair, nail, alimentary secretion, and finally to bone tissue containing 99% of the total body lead burden but turning over slowly. Therefore, the laboratory investigation of choice is whole blood lead while urine lead excretion rate should be used for monitoring chelation therapy. In this patient, the blood lead concentration was 3.15mmol/L (normal <0.48mmol/L).12 Other heavy metals were not elevated. His urine total protein concentration was less than 0.1g/L indicating the absence of significant renal damage. He had recently travelled to India with his Indian wife who gave him 5 different kinds of traditional medicinal drugs as aphrodisiacs. One of these 5 drugs was analysed to contain about 2800 ppm of lead (normal allowable limit <0.1).16 He was advised to stop all these drugs and was successfully treated with chelation therapy using 2,3-dimercaptosuccinic acid (DMSA).17 Local cases of mercury poisoning Like lead, mercury is an environmental toxin. Three forms exist naturally: elemental, inorganic, and organic, with methyl mercury representing the prototype of the organic form of mercury compounds. In western societies, mercurial compounds have been used historically as anti-microbial agent,18 especially in the treatment of syphilis.19,20 Not until 1955, calomel (mercurous chloride) was detected in teething powders and worm medication in the United Kingdom.21 It was responsible for the poisoning of a large number of children after dental treatment (Pink disease).22 Thereafter, the Minamata incident in Japan remains the best known example of massive mercury poisoning in humans due to excessive discharge of contaminated industrial wastes into the aquatic environment.23,24 Mercury poisoning in fungicide-users is an example of occupational risk. Over-exposure can also occur with industrial accidents resulting in pneumonitis due to its volatile nature.25 Mercury is known as 「汞」 or 「水銀」 in Chinese. A number of Chinese medicinal compounds contain inorganic mercury salts. The inorganic form can exist in more than 30 kinds of mineral and medicinal compounds as well as mixtures of compounds including mercury sulphide(辰砂,三方晶系;黑辰砂,等軸晶系),mercury-antimony ore(天然汞,硫汞銻礦),mercury-antimony-copper ore(汞黑幼礦),mercuric or mercurous chloride(降丹,水火丹,升汞,輕粉), mercury oxide (三仙丹,升丹,小升丹,靈藥,三白丹,三仙散,紅升,黃升,紅粉,升藥底)。 Calomel was commonly used as a cathartic because soluble mercuric ions were thought to inhibit the re-absorption of sodium and other ions in the intestines leading to retention of electrolytes and water in the bowel. Its aqueous solution has been used as a fungicide for the topical treatment of various skin diseases. It can be used topically with realgar for skin infection. In western medicine, all species of mercury are considered as toxic. Organic mercury is readily absorbed through our diet from contaminated seafood. This is the most common source of contacting mercury in daily life. Inorganic mercury is corrosive to the mucous membrane, while exposure to elemental mercury is usually by accident either in the industry, hospital, or through deliberate self-harm. After absorption, elemental mercury is ultimately converted in vivo by the action of catalase, to the active inorganic mercury ions (Hg2+) that bind avidly to the sulphydryl group of most proteins and enzymes where the active sites are situated and render them inactive. Mercuric ions also displace other divalent metal ions such as copper ion from active sites thereby inhibiting the oxidative-reductive reactions. Organic mercury is demethylated to mercuric ions, which will then exert the same toxic effects. Organic and elemental mercury are even more toxic as they readily pass through the blood-brain-barrier as well as placenta. Therefore, the foetus, babies, and developing children are at an increased risk of mental side effects. The acute load of mercury will be handled by our renal system. It is not surprising that the kidney is another target organ for damage. Tubular damage can result in proteinuria and even nephrotic syndrome.26 Therefore, urinary excretion rate is the most appropriate parameter for monitoring chelation therapy. Acute poisoning with elemental mercury will cause severe pneumonitis and adult respiratory distress syndrome. Elemental mercury is well absorbed through the pulmonary vasculature. Shock and acute renal failure will then follow 4-12 days after initial exposure. For acute poisoning with inorganic mercury, mucous membrane ulceration, excessive salivation, nausea, vomiting, abdominal pain, diarrhoea, lethargy, oliguria or even anuria may be resulted. Complications such as gastrointestinal bleeding, acute renal failure, and circulatory failure can sometimes result in fatality. In chronic poisoning with organic mercury and other mercury species, the signs and symptoms are less well demarcated. Mild symptoms like back and joint pain are very common. Neuropathy, mental disturbance, tunnel vision, etc, represent late and ominous signs of chronic intoxication. Therefore, the best marker for the laboratory diagnosis of acute and chronic mercury poisoning is whole blood mercury collected in certified specimen bottles. Case 4
A 38-year old housewife presented with a laboratory report showing markedly elevated
hair mercury content of 22.5mg/g (normal 11.6). Her family doctor had requested
the test because of her low back pain and previous occupation in an electroplating
factory 13 years ago. Other toxic metals such as lead, cadmium, arsenic and thallium
were within normal limits. She denied any colouring or permanent wave treatment
of hair before the sample was taken. On examination there was no psychosis, neuropathy,
dermopathy or arthropathy. However, her blood mercury (94nmol/L, normal
Further investigation The offending cream was found to contain an extremely high mercury content at 6.5% w/w, that is, 65,000 ppm (normal <1).27 The mercury species in this cream was found to be largely inorganic (95.3%). Interpretation A survey of mercury content for locally available beauty creams and cosmetics has been published.28 Consumers should be careful and selective in purchasing beauty creams and other cosmetics. They should prefer products that are manufactured in developed countries, where regulations for quality control and product labelling are more stringent before export. Price should not be the principal concern in their selection, and they should be aware of fake or pirated products. Family physicians and other specialists should be aware that patients might be exposed to widely available and easily purchased cosmetics and other products that are adulterated or contaminated with heavy metals such as mercury, lead, cadmium, and arsenic, and be alert to the possibility of chronic heavy metal poisoning with vague and non-specific signs and symptoms. In suspected cases, they should refer the patients for heavy metal analysis and other appropriate laboratory investigations including a complete blood count and renal function test. Case 5 A 5-year old Chinese boy of healthy unrelated parents presented on two occasions: (i) initially with oral ulceration. Herpetic ulceration was diagnosed and confirmed by the isolation of herpes simplex virus (HSV) type 1 from his tongue swab. The lesion improved after treatment with a 5-day course of oral acyclovir; and (ii) sudden onset of motor tics consisting of eye blinking, head turning, and shoulder shrugging 5 weeks later. On examination, there were episodes of motor tics as described. No skin rash or desquamation on the palms and soles was noted. There was a small healing ulcer at the tip of his tongue. His speech and gait were normal. Cardiovascular, respiratory, abdominal, and neurological examinations did not reveal any abnormalities. Sensory and motor nerve conduction velocities were reported to be normal. Other investigations including complete blood count, renal function tests and electrolytes, liver enzymes, immunoglobulins, complements, as well as urinalysis and toxicology screening were all normal. Serum anti-neuronal antibody and anti-streptolysin O titre were not elevated. Electroencephalography, cranial computerised tomography, and magnetic resonance imaging of the brain were also normal.
However, his blood mercury concentration was found to be 83 nmol/L (normal
Interpretation This patient had been on a normal unrestricted diet and there was no history of excessive seafood consumption. On further questioning, his mother admitted that the patient had been given a Chinese medicinal mouth spray, named "Watermelon Frost (西瓜霜)", 20 times daily for 4 weeks preceding the second admission. The spray was believed to be useful in controlling pain and healing mucosal wounds. After abstinence from the use of the offending mouth spray for 4 weeks, blood mercury concentration had decreased to below the normal reference interval with resolution of his motor tics.29 The mercury content of the spray was 878 ppm (normal allowable limit <1).27 The mercury species in this mouth spray was again found to be largely inorganic (98%). Mouth ulceration is one of the most common problems encountered in general practice. Most mouth ulcers are caused by Herpes Simplex Virus Type 1. Healing usually occurs with time. Acyclovir can be prescribed as an anti-viral therapy in severe cases but it is quite expensive. In our local population, alternative medicine are often used which could be adulterated with western medicine purposefully or contaminated with unwanted substances such as heavy metals unintentionally during sub-standard manufacturing process. Family physicians and other specialists should always be alert of the signs and symptoms as well as the possibility of heavy metals poisoning. Industrial over-exposure or contamination of cadmium and cobalt at the workplace Unlike lead or mercury, industrial exposure of cadmium and cobalt is a recently noticed phenomenon. The processes of smelting and refining of zinc and lead ores produce dust and vapour laden with cadmium. Electroplating, soldering, brazing and the disposal of industrial waste also generate cadmium-rich vapour and dust. Other sources of cadmium are from diet including shellfish, animal kidneys, and tobacco. The gastrointestinal absorption of cadmium is variable depending on the intake of other divalent nutritional metals as they share the same carrier for absorption. Once absorbed, cadmium is transported within the plasma to liver where it is combined with metallothionein which will then be re-distributed to the renal cortex slowly via glomerular filtration and tubular reabsorption. Whole blood cadmium concentration is therefore a better index of exposure as urine cadmium excretion is usually normal until renal damage has occurred. Exposure of inorganic cobalt can occur during production of tungsten carbide materials, manufacture of alloys and pigments, and corrosion of cobalt-alloy joint prosthesis. Cobalt can cause cardiomyopathy resulting in fulminating heart failure and polycythaemia. Hard metal interstitial lung disease can also occur following occupational exposure of cobalt powder or vapour. Unlike cadmium, inorganic cobalt exposure requires urinary assessment as it is rapidly excreted in urine within 2-3 days after initial exposure.30 The Occupational Safety & Health Branch of the Labour Department of Hong Kong as well as the Occupational Safety & Health Council have jointly published guidance notes on medical examinations for workers engaged in hazardous occupations in industrial undertakings in November 2003. Workers at risk of contacting toxic metals are recommended to have regular and appropriate medical check-up including chest radiograph, lung function, audiometric, blood, and urine tests for the assessment of industrial over-exposure and early detection of complications. The panel of heavy metals includes arsenic, cadmium, manganese, lead, and mercury.31 Case 6 Three workers in a battery manufacturing factory had their yearly laboratory assessment:
Interpretation All three workers had a significant exposure of cobalt but only two of them had significant exposure of cadmium. It is important to note the smoking habit of these workers in order to interpret the blood cadmium results correctly because the reference intervals for smokers and non-smokers are totally different. All workers showed normal urine b-2-microglobulin to creatinine ratio indicating the absence of renal damage even though they had had a significant exposure. Control samples were collected by instructing workers to decant double distilled water standard using their own hands to specimen bottles for excluding the possibility of false positive result due to environmental contamination during sample collection. Conclusion In conclusion, the following approach is recommended for investigating patients with suspected heavy metal poisoning: (i) Identification of possible source(s) of exposure Occupational, drug and dietary histories as well as personal habits are important information to note.1 If possible, obtain the remains of the suspicious drug, cosmetics, and food for future analysis. (ii) Laboratory diagnosis Clinical manifestation of heavy metal poisoning is often protean with signs and symptoms that are often subtle and ill-defined. Proper diagnosis depends very much on the clinical acumen of the attending clinicians and a thoroughly taken medical history. In the absence of probable cause such as occupational and/or accidental environmental exposure, broad-spectrum screening for trace elements and other analytes is inappropriate and would rarely be indicated in general practice.33 Proper laboratory investigations serve to confirm the diagnosis. If necessary, analysis of the offending drug, cosmetics or food can also be performed.26,28,29 However, contamination is a major challenge to laboratories performing trace element analysis. Quality results require good pre-analytical preparation. Obtaining instructions for sample collection and certified specimen bottles from a reputable laboratory that has participated in regular quality assurance programme for trace element analysis is essential. (iii) Assessment of complications Renal toxicity with tubular cell damage resulting in significant proteinuria is common. Urine retinol binding protein or b-2-microglobulin normalised to creatinine can be requested as an objective assessment of tubular proteinuria. Central nervous system is another common site affected by heavy metals such as lead and mercury after they have crossed the blood-brain-barrier. Nerve conduction study can be requested as a non-invasive but objective measurement of the neurotoxic effects of heavy metal deposition. (iv) Chelation therapy This depends very much on the severity, toxic signs and symptoms. Advantages and disadvantages of the chelation therapy have to be considered thoroughly and discussed with the patient since chelation therapy may have deleterious side effects.34 Key messages
Michael H M Chan, MBChB(CUHK), FRCPA, FHKCPath, FHKAM(Pathology)
Deputising Senior Medical Officer, Robert C K Cheung, PhD(CUHK) Scientific Officer (Medical), Eric C W Lam, MBChB(CUHK), PhD(CUHK), FRCPA, FHKAM(Pathology) Associate Professor, Christopher W K Lam, PhD(S'ton), FRSC(UK), FACB(USA) Chairman and Chief-of-Service, Department of Chemical Pathology, The Chinese University of Hong Kong, Prince of Wales Hospital. Albert Y W Chan, MBChB(Glasg), MD(CUHK), FHKCP, FHKCPath Consultant Chemical Pathologist, Department of Pathology, Princess Margaret Hospital. Tony W L Mak, MBChB(CUHK), MBA, FRCPA, FHKAM(Pathology) Consultant, Department of Clinical Pathology, Tuen Mun Hospital. Anthony C C Shek, MBBS(HK), FRCPath, FRCPA, FHKAM(Pathology) Consultant, Department of Pathology, Queen Elizabeth Hospital. Sidney Tam, FRCP(Edin), FRCPA, FHKAM(Medicine), FHKAM(Pathology) Head and Consultant, Division of Clinical Biochemistry, Department of Pathology, Queen Mary Hospital. Correspondence to : Prof Christopher W K Lam, Department of Chemical Pathology, The Chinese University of Hong Kong, Prince of Wales Hospital, Shatin, N.T., Hong Kong. Email : waikeilam@cuhk.edu.hk
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
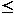 45)17 and urine mercury excretion (345
nmol/day, normal 50)12 were elevated, while other biochemical and haemato-logical
investigations were normal. As she consumed a typical Chinese diet containing fish
caught near local industrial areas, she was advised to avoid seafood. Four weeks
later her blood mercury concentration had decreased to 77nmol/L, which was still
high. She volunteered that she has been using a skin-whitening cosmetic cream twice
daily over her face for several months. She was also advised to stop using this
cream. Two weeks later, her blood mercury concentration had decreased markedly to
25 nmol/L, which was well below the upper reference value, while the daily urine
mercury output was still high (313 nmol/day), indicating ongoing excretion of the
mercury load.
45)17 and urine mercury excretion (345
nmol/day, normal 50)12 were elevated, while other biochemical and haemato-logical
investigations were normal. As she consumed a typical Chinese diet containing fish
caught near local industrial areas, she was advised to avoid seafood. Four weeks
later her blood mercury concentration had decreased to 77nmol/L, which was still
high. She volunteered that she has been using a skin-whitening cosmetic cream twice
daily over her face for several months. She was also advised to stop using this
cream. Two weeks later, her blood mercury concentration had decreased markedly to
25 nmol/L, which was well below the upper reference value, while the daily urine
mercury output was still high (313 nmol/day), indicating ongoing excretion of the
mercury load.