December 2006, Vol 28, No. 12 |
Update Article
|
||
Investigation of ischaemic chest pain: which tests to choose?John T H Wong 王泰鴻,Andy W K Chan 陳偉光 HK Pract 2006;28:530-539 Summary Chest pain is a common symptom that prompts patient to present to a clinician. Clinicians not specializing in cardiology may become confused and overwhelmed by the wide range of newer investigations for the assessment of suspected ischaemic chest pain. These include multislice computed tomography, cardiac magnetic resonance imaging and coronary angiography complemented with intravascular ultrasound. Together with conventional tests like exercise electrocardiography, radionuclide imaging and stress echocardiography, they all provide the precise diagnosis with good to excellent accuracy and the severity of the underlying coronary artery disease. Some investigations allow deeper insights into the mechanism of various aspects of coronary artery disease and contribute important implications in the diagnostic and therapeutic strategies in treating coronary artery disease. Therefore, by choosing a series of most suitable tests for patients suffering from suspected ischaemic chest pain will allow the identification of those who are most at risk for adverse cardiovascular events and facilitate in deciding the most appropriate interventions and eventually improve their outcomes. 摘要 胸痛是促使病人求醫的常見病徵。作為非心臟專科醫生,可能會對不同種類,日新月異的冠心病醫療檢查方法感到迷惑,難以取捨。這些包括多層薄片電腦掃瞄、心臟磁共振成像,以及心臟血管造影術輔以血管內超聲波檢查。還有傳統的測試:運動心電圖、放射性核素成像和壓力心臟超聲波。它們都能為冠心病提供精確診斷,並對其嚴重程度作出評估。一些檢查更可讓我們進一步了解冠心病的各種機制,對診斷和治療提供很大的幫助。因此選擇適當的測試可辨認較為高危的心臟血管病病人,從而採用恰當的治療以達到更佳的效果。 Introduction Chest pain is a very common presenting symptom in patients suffering from coronary artery disease (CAD). Prompt and efficient assessment of patients with chest pain is central to any strategy aiming to relieve their symptoms and improve their outcomes by providing them with appropriate treatment. Moreover, the investigation of patients with chest pain occupies a considerable amount of the resources of both general practitioners and cardiologists. Routine workup by taking a detailed history to elicit features of ischaemic chest pain together with careful physical examination and then investigating using well-established procedures such as exercise electrocardiography (ECG), functional imaging (e.g. radionuclide imaging, stress echocardiography) and the "gold standard" test - coronary angiography, are well described in any textbook of medicine or cardiology. However, to formulate a service that combines these principles and to deliver them in a timely, accurate and cost-effective format is a major challenge to most clinicians. Furthermore, the emergence of more recent cardiovascular imaging modalities, namely intravascular ultrasound (IVUS), multislice computed tomography (MSCT) and cardiac magnetic resonance imaging (CMRI) also provide new perspectives in the assessment and diagnosis of CAD and offer the clinicians more options in choosing the most appropriate test for each individual patient. In this review article, the conventional and newer procedures that are used to investigate and diagnose CAD and their current applications will be briefly discussed. Non-invasive modalities Exercise electrocardiography Exercise ECG is usually the initial test of choice for the evaluation of patients with suspected cardiac chest pain. The indications of exercise electrocardiography to diagnose obstructive CAD as recommended by AHA/ACC 2002 Guideline Update for Exercise Testing1 are shown in Table 1. For obvious reasons, exercise ECG is inappropriate in patients who are unable to exercise (e.g the presence of peripheral vascular disease or osteoarthritis of the knees, etc.) and is of little diagnostic usefulness in patients with particular ECG abnormalities at rest (Table 1). Exercise ECG test is frequently performed in the outpatient settings and should be supervised by a physician. During the test, the physician should be nearby and available in case of emergency.2-3 Exercise ECG is a relatively safe procedure with a minor complication rate of about 2 % (mainly arrhythmias and hypotension).4 The rate of acute myocardial infarction or death is about 1 in 2500 tests.5 Table 1: 2002 Exercise testing guideline recommendation for diagnosing CAD
Data from a meta-analysis involving 147 studies and 24,074 patients indicated that as compared to coronary angiography, exercise ECG had a sensitivity of 68% and a specificity of 77% for detection of CAD.7 However, results varied markedly among studies, reflecting heterogeneity of criteria for abnormal results and various degrees of severity of coronary disease in the study populations. In patients without a high risk of extensive coronary diseases, the sensitivity of exercise ECG may be as low as 45%.8 Exercise testing is less sensitive in women than in male, and some data suggested that it may also be less specific.6 The interpretation of the results of an exercise ECG test as positive or negative on the basis of ECG changes alone, without consideration of other important parameters like patient's symptoms, exercise capacity and changes in blood pressure and heart rate, is to be avoided. One approach to integrating data from exercise ECG is through the use of Duke treadmill score (Table 2).9 This treadmill score is useful in helping clinicians to determine prognosis and decide whether to refer patients with suspected coronary disease for coronary angiography.
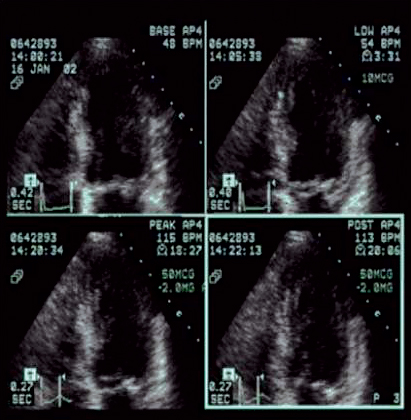
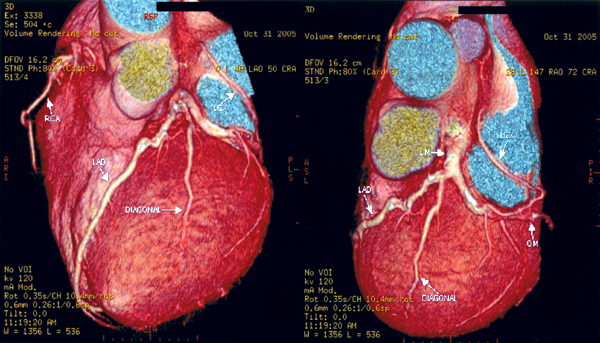
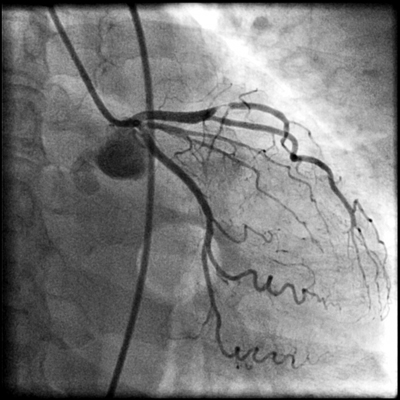
Radionuclide imaging is a commonly used imaging technology in assessing patients with known or suspected ischaemic chest pain. Most radionuclide imaging in cardiology applications use SPECT to reconstruct anatomical slices of discrete thickness in standardized views. More complicated scanners for positron-emission tomography (PET) use multiple rings of stationary detectors that detect emissions from tracers, which can be used to determine regional myocardial blood flow and metabolic processes. However, PET scanners are more costly than those of SPECT and are not widely available for routine use. SPECT requires the use of radioactive tracers. These tracers are labelled either with thallium TI 201 or technetium Tc99m and are used to assess myocardial perfusion and viability. The uptake of these agents by myocardial cells depends on both the perfusion and viability of that region of myocardium. The initial distribution of these tracers is proportional to myocardial blood flow, whereas so-called redistribution images, which are obtained 3-4 hours later, reflect myocardial viability and are independent of flow. A myocardial defect on an initial scan that subsequently resolves is an indicator of myocardium that is viable but ischaemic. A defect that is apparent on both scans suggests a region of myocardium that has died, usually as a result of previous myocardial infarction. Ischaemia can be provoked with physical exercise or pharmacological agents. Dobutamine is a positive inotropic agent that provokes ischaemia by increasing myocardial workload, whereas adenosine and dipyridamole are vasodilators that unmask coronary stenoses by "coronary steal", i.e. by diverting blood flow to coronary arteries that are not diseased. All three drugs are useful in patients who are unable to perform physical exercise. As compared to coronary angiography, the sensitivity and specificity of SPECT in detecting CAD are 88% (range from 73-98%) and 77% (53-96%) respectively.10 The diagnostic performance of technetium-based agents is similar to that of thallium.6 The diagnostic yield of the above pharmacological agents with respect to radionuclide perfusion imaging is similar and is also similar to that obtained with physical exercise.11 Nevertheless, dobutamine has not been studied as extensively as the two vasodilators; it is recommended for use when there are contraindications to the use of adenosine or dipyridamole (e.g. allergy, asthma, heart block, etc.).6 Same as exercise ECG, concurrent use of beta blockers and other anti-ischaemic medications may reduce the sensitivity of radionuclide imaging for the detection of CAD. Such agents should be stopped 4-5 half-lives before testing.6 Radionuclide imaging also provides prognostic information as from exercise ECG in both men and women.12-14 However, same as exercise ECG, radionuclide perfusion imaging is less accurate in women than in men. Some studies suggested that the better imaging properties of technetium 99m sestamibi may make it a superior agent for the evaluation of obese patients or women with large breast or breast implants.15 Stress echocardiography Stress echocardiography is another imaging modality available for the investigation of ischaemic chest pain. It involves either exercise or pharmacological agents to induce ischaemia.16 Echocardiographic images acquired while patient is at rest and immediately during pharmacological stress using dobutamine or dipyridamole (or immediately after stress for patient undergoing exercise treadmill stress) are compared side by side (Figure 1). A study is considered positive if regional wall motion abnormalities develop with stress in previously normal segment or deteriorate in a segment that was already abnormal at baseline. In addition to providing information on the location and extent of jeopardized myocardium, this test also gives insight into left ventricular and valvular functions. Figure 1: Images from a stress echocardiographic study. Regional wall motion abnormalities are always better shown by digital cine loops. The diagnostic performance of stress echocardiography varies widely, with a sensitivity of 76% (range from 40-100%) and a specificity of 88% (80-95%) for the detection of CAD while using coronary angiography as the "gold standard".10 As compared to radionuclide perfusion imaging, data had shown that the sensitivity of stress echocardiography is slightly lower.10,17 As is true of other non-invasive test for ischaemia, the test has a higher sensitivity for multivessel disease (~90%) than single vessel disease (56%).6,18 Pooled data had found that exercise echocardiography is more specific than exercise SPECT.19 Dobutamine and dipyridamole stress echocardiography have similar diagnostic yields.20 Apart from being used as a diagnostic test for ischaemic chest pain, stress echocardiography can further provide important prognostic information and had been validated by long-term studies. Among medically treated patients, the 4-year rate of infarction free survival was lower in patients with positive tests than in those with negative tests (87% vs 97%).18 These data suggested that patients with positive tests have a better prognosis if they are treated invasively.18 A negative exercise echocardiography is reassuring, as the rate of survival without cardiac events is 99.2% at 1 year and 97.4% at 3 years.21 Rapid development of stress echocardiography imaging using ultrasound contrast agents (basically made up of microbubbles able to traverse pulmonary and systemic circulation) now may allow simultaneous assessment of both myocardial function and perfusion.22-23 Although myocardial contrast stress echocardiography is not currently used as a routine test for the assessment of CAD, it is likely that it will become an integral part of stress echocardiography in the future. Multislice computed tomography (MSCT) Cardiac MSCT have gained enormous popularity as a test for the assessment of ischaemic chest pain and a non-invasive alternative to coronary angiography over the recent years. With the swift advances of technology in MSCT (newer scanners have 16 or 64 detectors), there are significant improvements in its speed and spatial resolution (as high as 0.4 x 0.4 x 0.5 mm) as compared to those of previous generations. Together with the ability to time the scan by synchronizing with the cardiac cycle using electrocardiogram (ECG), current MSCT has minimized the cardiac-motion artifacts and allows for precise analysis of cardiac anatomy (Figure 2). Figure 2: 3D reconstructed MSCT film showing proximal LAD and LCx stenosis The performance of MSCT coronary angiography involves radiation exposure (as high as 10-20 mSV) and requires the administration of intravenous contrast; a patient who is allergic or intolerant to such agents (e.g. significant renal impairment and pregnancy) should avoid this examination. Breath holding of short duration (~ 5-10 second) is also mandatory. Inability to hold the breath will increase the likelihood of poor scan quality due to respiratory motion artifacts. Regular heart rhythm and slow heart rate (heart rate ~ 60-80 bpm) are also crucial for capturing optimal images of the coronary arteries. MSCT coronary angiography is now used clinically to assess coronary vessels and bypass grafts non-invasively. Numerous studies have been conducted on the accuracy of MSCT angiography in cases of known or suspected CAD.24-28 Comparing sensitivity and specificity of MSCT in detecting coronary lumen obstruction of > 50% with invasive coronary angiography ranged from 82-100% and 78 to 98% respectively. The real strength of MSCT appears to be its high negative predictive value which, in the most recent studies, ranged from 95-99% among patients at intermediate-to-high risk of CAD. More recently, appropriateness reviews were conducted by the American College of Cardiology Foundation (ACCF) together with key specialty and subspecialty societies for the application of MSCT coronary angiography in the evaluation of chest pain and had determined that such test is appropriate in patients who had any of the following clinical features: intermediate pre-test probability of CAD, uninterpretable resting ECG (see Table 1), inability to exercise, previously uninterpretable or equivocal stress test (exercise, perfusion, or stress echo).29 There is no long-term data regarding outcomes for patients who have negative tests, although there will be forthcoming shortly. Therefore, patients who have chest pain but are at low-to-moderate cardiovascular risk or equivocal stress tests are ideal candidates for MSCT coronary angiography. Indeed, for the particularly anxious patient with atypical chest pain, the combination of a low-risk stress test and a negative MSCT coronary angiogram is an impressive tool for reassurance. Electron beam computed tomography (EBCT) is another subtype of computed tomography which had long been available for detection of coronary calcium deposits as a surroagte marker for coronary atherosclerosis. EBCT does not require iodinated contrast and the only identifiable component of the coronary artery is calcium within an atherosclerotic plaque. While the detection of high calcium content within the coronary artery (expressed as high calcium score) is sensitive for the presence of significant CAD, the ACC/AHA Consensus Panel did not recommend such test for diagnosing obstructive CAD because of its low specificity.30 In short, calcium scoring by EBCT is probably better used as a risk factor indicator for CAD rather than as a diagnostic test. Cardiac magnetic resonance imaging (CMRI) Rapid progress has been made in CMRI over the past 10 years and has firmly established itself as a reliable and clinically important technique for the assessment of CAD by means of coronary magnetic resonance angiography (CMRA) and first-pass contrast enhanced CMRI. CMRI is non-invasive, has high spatial resolution and does not use ionising radiation. MRI is based on the principle of nuclear magnetic resonance. In very simplistic terms, images are derived from signals by protons in body water. Such proton behaves like a small magnet and will therefore align parallel and anti-parallel to the direction of the primary magnetic field, with a small excess of parallel protons that gives rise to a net magnetisation vector. By application of a secondary temporary radiofrequency pulse, this net vector can be altered and once the pulse is discontinued, the vector reverts back to its former position, releasing signal in the form of radiowaves. Further elaboration of the underlying mechanism and techniques of CMRI is beyond the scope of this article and had been described elsewhere. Currently, CMRA is much less commonly used than MSCT angiography to detect CAD as there is limited evidence that CMRA can accurately do so.31 Indeed, CMRA had been judged to be an inappropriate test for the evaluation of patients with chest pain except in case of suspected coronary anomalies by ACCF and other related specialty and subspecialty societies.29 On the other hand, first-pass perfusion CMRI has been reported to have high diagnostic accuracy for detection of CAD. First-pass perfusion CMRI assesses myocardial perfusion reserve (an indirect measures of coronary flow) by establishing the signal intensity-time curves before and after pharmacologically induced hyperaemia. The sensitivity, specificity and diagnostic accuracy are 90%, 83% and 87% respectively for the detection of CAD as compared to coronary angiography.32 CMRI perfusion reserve improves after revascularization.33 Further developments in imaging protocol (multislice techniques with fast near-real-time sequences) had improved spatial and temporal resolution of the scan and have resulted in near-complete myocardial coverage, making first-pass CMRI perfusion a viable alternative to scintigraphy. According to the latest guidelines,29 perfusion CMRI is an appropriate test in assessing patients with chest pain who had any of the following: intermediate pre-test probability of CAD, uninterpretable ECG (see Table 1) or inability to exercise. Its use is also appropriate in the evaluation of the functional significance of an intermediate coronary stenosis shown on MSCT or conventional invasive coronary angiography. Invasive modalities Coronary angiography Invasive coronary angiography was first introduced in the late 1950s and the utilization of coronary angiography had increased exponentially since then. Coronary angiogram is performed by inserting a specialized, preshaped catheter into a major artery percutaneously (most commonly via the superficial femoral artery a few centimetres below the mid-point of inguinal ligament), and with minimal manipulation, the catheter will then engage into the coronary ostia. Contrast agent is then injected into the coronary arteries and thus become opacified. The images of the coronary arteries will then be captured by the digital X ray camera (Figure 3). More than 4 million coronary angiograms were performed in the United States each year. With current technology, it delivers digital images of superior spatial (0.2mm) and temporal (5ms) resolution. Coronary angiography is the "gold standard" test for diagnosing CAD. Figure 3: A coronary angiogram showing a critical lesion in the first diagonal artery Coronary angiography requires high level of technical competence and specific equipments, which makes it relatively expensive. It is associated with mild patient discomfort during the procedure and the risk of serious complications is <1/1000,34 hence restricted to a selected group of patients. Furthermore, coronary angiography has its own limitations; it is basically a "lumenogram" and provides relatively little details in regard to the nature and characteristics of the coronary plaques. Because of this problem, the use of complementary intravascular ultrasound during coronary angiography had greatly facilitated the cardiologist to measure and characterize coronary vessels and atherosclerotic plaques. Intravascular ultrasound (IVUS) IVUS is an invasive procedure which is performed during cardiac catheterization using miniature ultrasound probes mounted on the tip of a specialized coronary catheter. The IVUS probe emits high ultrasound frequencies and the ultrasound signal reflected from arterial wall structures is used to generate a grey scale image. The probe is placed beyond the target lesion site and the ultrasound catheter is then slowly pulled back during continuous imaging. This results in a series of images displaying 360o cross-sectional views of layers of the coronary artery (intima, media and adventitia) as well as the lumen. Extensive experience has shown that IVUS is a safe, accurate and reliable method for assessment of the severity and morphology of coronary lesions.35 Using IVUS, some morphological features of atherosclerotic coronary plaques can be readily recognised. For example, soft plaque is mainly composed of echolucent tissue and correlates with a high lipid content and necrotic tissue; while calcific plaque is highly echogenic because of its calcium content. Other structure that can be viewed with IVUS includes intraluminal thrombus which is usually seen as a mass in the lumen, and intimal hyperplasia which appears as barely echogenic tissue within a stent. The main clinical application of IVUS is in the placement of stent during percutaneous coronary intervention (PCI). The use of IVUS immediately before stent placement would provide strategic information on the exact length and size of the stent needed. After placement of the stent, IVUS also helps the operator in deciding whether additional balloon inflation is required if the stent was suboptimally expanded, and to look for post-stenting complication like stent edge dissection in which case additional stents may be needed to cover the new lesion. More importantly, the use of IVUS can improve PCI outcomes. Several studies had demonstrated that IVUS guided stenting using bare-metal stent had better lumen dimensions, with some reduction in restenosis and target lesion revascularization at follow-up as compared to that guided by conventional angiography.36-40 Conclusion Various investigations available for the assessment of suspected ischaemic chest pain, whether with conventional tests like exercise ECG, radionuclide imaging and stress echocardiography, or with newer imaging modalities like MSCT, CMRI or coronary angiography complemented with IVUS, provide the precise diagnosis with good to excellent accuracy and the severity of the underlying CAD. Some investigation allows deeper insights into the mechanism of various aspects of CAD (e.g. IVUS in the understanding of ISR) and its implications in the diagnostic and therapeutic strategies in treating CAD. To sum up, it is hoped that by choosing a series of most suitable tests (as recommended by the authors - see Figure 4) will allow the identification of those who are most at risk for adverse cardiovascular events and facilitate in choosing the most appropriate interventions and eventually improve their outcomes. Figure 4: Suggested flowcharts for the assessment of patients with suspected ischaemic chest pain 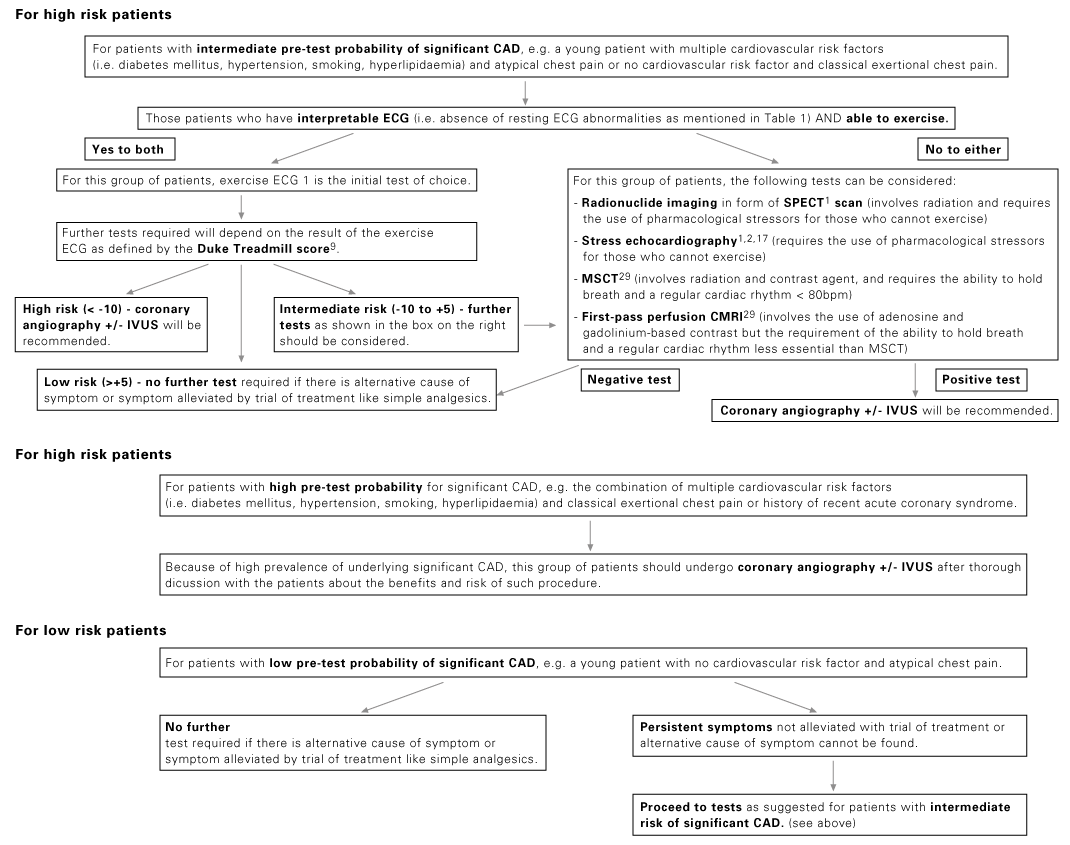
Key messages
John T H Wong, MBBS (Hons)(HK), MRCP (UK), FHKCP, FHKAM (Medicine)
Andy W K Chan, MBBS (HK), FRCP (Lond), FHKCP, FHKAM (Medicine) Correspondence to: Dr Andy W K Chan, Division of Cardiology, United Christian Hospital, Kwun Tong, Kowloon, Hong Kong. References
|
|||