Summary
Most glaucoma patients have no symptoms and quite
often their disease are only suspected or confirmed during routine eye
screening. It is a slowly progressive condition in which the retinal
nerve fibers degenerate. If untreated, blindness is almost invariable
although the time course and speed vary. Up to the current moment, intraocular
pressure reduction is the only effective mechanism of treatment by which
the disease process can be slowed or stopped. The role of neuro-protection
medications is still uncertain.
摘要
大多數的青光眼病人沒有任何症狀,通常是眼科常規檢查時被懷疑或確診患有該病。青光眼是視網膜神經慢性的進行纖維退變。若未經治療,就會導致失明,雖然病程和失明的速度有所不同。到目前為止,降低眼壓(IOP
reduction)是唯一可減緩或終止疾病過程的有效機制。神經保護藥物的作用尚不清楚。
Introduction
Glaucoma is a heterogeneous group of ocular diseases
that manifest as a progressive optic neuropathy and visual loss.1,2
Visual field loss and even irreversible blindness occur if glaucoma
is not appropriately diagnosed and treated. Despite the availability of
intraocular pressure (IOP) lowering agents that may arrest or slow disease
progression, glaucomatous blindness still occurs.
Acute angle-closing glaucoma can be considered to be a separate
entity in which patients may not have any visual field defects or high
IOP before the attack. It happens in individuals who have occludable angles
as a result of naturally-occurring or acquired abnormal anatomy of the
angle structures. During the acute attack, patients have symptoms of severe
ocular pain and headache, blurring of vision and nausea and vomiting as
a result of rapidly increasing IOP. Even with effective medical and laser
treatment, some of these acute cases may eventually end up in some forms
of chronic angle- closure glaucomas, the consequences of which may be
similar to those of the chronic open-angle types. The main focus of this
paper concentrates on the various clinical aspects of the latter.
The nature and burden of
glaucoma
Most glaucomas are insidious in nature, with no symptoms
or warning signs prior to advanced visual field loss.1,2 At
least half of the patients with open-angle glaucoma are not receiving
treatment because the disease is undiagnosed.1,3-6 Among treated
glaucoma patients, poor compliance is a major obstacle.2,7,8
It is estimated that 6.7% of the 67 million people with glaucoma worldwide5
- and 120,000 of the 3 million people afflicted with it in the United
States1 - are blind as a result.
Glaucoma is a significant public health problem. In
the United States alone, glaucomatous blindness costs an estimated US$1.5
billion annually in Social Security benefits, lost income tax revenues,
and healthcare expenditures.9 Added to the economic burden
imposed by blindness is the impact on patients' lives. Even before blindness
occurs, patients diagnosed with and treated for glaucoma may be troubled
by treatment inconvenience and the fear of vision loss.
Epidemiology of glaucoma
Glaucomatous optic neuropathy is most prevalent among
people of African origin, and least prevalent in full-blooded Australian
aborigines;10 Asian populations have rates intermediate between
theses two groups. European- and African- derived peoples suffer predominantly
from primary open-angle glaucoma (POAG), whereas rates of primary angle-closure
glaucoma (PACG) are higher among East Asians than in other populations.
Glaucoma as a cause of blindness
Population surveys in Mongolia found glaucoma to be
the cause of 35% of blindness in adults (cataract being the cause of 36%
of blindness).11 Among Chinese Singaporeans, 60% of adult blindness
was caused by glaucoma.12 Cautious extrapolation of these data
suggests that around 1.7 million people in China suffer blindness caused
by glaucoma. PACG is responsible for the vast majority (91%) of these
cases.13 Secondary glaucoma is the most common cause of uniocular
blindness.
Glaucoma is the leading cause of registered, permanent
blindness in Hong Kong (23%).14 In Japan, diabetic retinopathy
(18%), cataract (16%) and glaucoma (15%) are the leading causes of blindness.15
Risk factors for glaucoma
Apart from high IOP's, advancing age is the single most
consistent risk factor for all types of glaucoma.12,16-23 A
positive family history is also a risk factor for glaucoma.24,25
Female gender is recognized as a major predisposing factor toward the
development of PACG.12,20,23 There is little clear evidence
to support a gender difference in POAG. Those of Chinese ethnic origin
are at a higher risk of developing angle-closure glaucoma than those of
Malay descent and South Indian people.16,17 All the above
and in particular raised IOP have been shown by Foster et al in
a recent Singaporean Study to be risk factors for glaucomatous optic neuropathy
in Chinese people.26
A shallow anterior chamber has long been recognized
to be a factor that predisposes toward angle-closure.27 The
depth of the anterior chamber reduces with age and tends to be shallower
in women than in men.28,29 There may also be an association
between myopia and POAG.30 Other risk factors are diabetes
mellitus, hypertension and a thin central corneal thickness (<0.5mm).
Intraocular pressure and glaucoma progression
As mentioned above, glaucoma does not refer to a single
disease entity, but rather to a group of diseases that have certain common
features, including high IOP (too high for the continued health of the
eye), cupping and atrophy of the optic nerve head, and visual field loss.
IOP is determined by three factors: (a) the rate of
aqueous humor production by the ciliary body, (b) the resistance to aqueous
outflow across the trabecular meshwork - Schlemm's canal system, and (c)
the level of episcleral venous pressure. In most cases, increased IOP
is caused by increased resistance to aqueous humor outflow; in a minority
of cases, elevated IOP is caused by increased episcleral venous pressure.
Although the causes of glaucoma are still unknown, studies
suggest controlling IOP slows the risk of disease progression.31,32
In fact, elevated IOP is considered to be one of the most important risk
factors for glaucoma.2,3,6
Classification
The many types of glaucoma are classified, primarily,
as being of (a) the open-angle or (b) angle-closure type, according to
the manner in which aqueous outflow is impaired. In open-angle glaucoma,
the elevation in IOP is caused by increased resistance in the drainage
channels; whereas in angle-closure glaucoma the obstruction to aqueous
outflow is caused by closure of the chamber angle by the peripheral iris.
Figure
1a: Normal aqueous flow in the anterior and posterior chambers
|
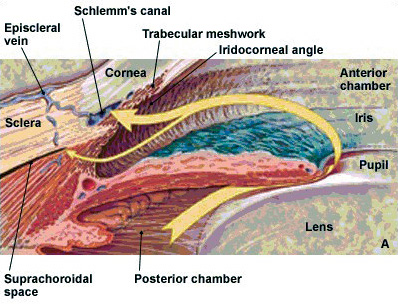 |
Figure
1b: The flow's main resistance is at the trabecular meshwork
in cases of open-angle glaucoma |
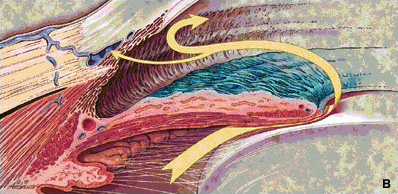 |
Figure
1c: In cases of pupil block, the iris bulges forward and the
angle is closed |
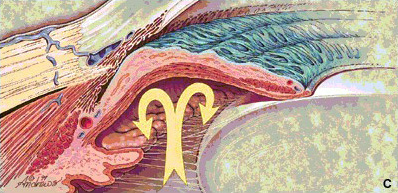 |
Further classification describes the disorder as (a)
primary or (b) secondary depending on the absence or presence of associated
factors contributing to the IOP rise (e.g., presence of proliferative
diabetic retinopathy and uveitis, use of topical steroids and following
trauma).
In some cases, the age of the patient at the onset of
glaucoma is also taken into consideration and the condition is then described
as congenital, infantile, juvenile, or adult accordingly.
Assessment
A full assessment will be preformed by an ophthalmologist
once he/she suspects the patient is suffering from glaucoma. The aims
of the initial assessment are:
| |
- |
to determine whether or not glaucoma is
present; or likely to develop (i.e., assess risk factors) |
| |
- |
to exclude or confirm alternative diagnosis |
| |
- |
to identify the underlying mechanism of
damage, so as to guide the choice management |
| |
- |
to begin planning a strategy of management
|
| |
- |
to identify suitable forms of treatment;
and to exclude those which are inappropriate. |
| |
In particular, the following
areas will be recorded: |
| |
- |
Level of IOP |
| |
- |
Gonioscopy (Angle status) |
| |
- |
Optic nerve head morphology |
| |
- |
Perimetry |
Intraocular pressure
The IOP is measured by various kinds of tonometers,
the gold standard of which is called Goldmann Tonometer. It is usually
a kind of slit-lamp mount instrument and designed in a way that the IOP
can easily be measured at the same time as the routine slit-lamp biomicroscopic
eye examination. Unfortunately there is no safe safety-margin below which
any eye can be free of glaucomatous damage. The figure of 21.5 mmHg is
a by-product of statistics (mean+/-standard deviations) using old Western
data base. However, in general, the higher the IOP is, the more is the
likelihood of the presence of glaucomatous damage to an eye.
Gonioscopy
Gonioscopy is crucial for the proper diagnosis of glaucoma.
Furthermore, it is essential for glaucoma treatment in the angle (e.g.,
laser trabeculoplasty). Gonioscopy should be performed on all patients
with glaucoma, on all glaucoma suspects, and on all individuals thought
to have narrow angles.
Proper management of glaucoma requires that the clinician
determine whether the angle is open or closed. In angle-closure, the peripheral
iris obstructs the trabecular meshwork, (i.e., the meshwork is not visible
on gonioscopy). The width of the angle is determined by the site of insertion
of the iris on the ciliary face, the convexity of the iris, and the prominence
of the peripheral iris roll. If the angle between the peripheral iris
and the trabecular meshwork exceeds 20o, angle-closure is unlikely.
Optic nerve head morphology
Ophthalmoscopy is used to examine the inside of the
eye, especially the optic nerve. Glaucomatous damage typically appears
as cupping or increased cup-to- disc area ratio. The cup is classically
defined as the white central part of the optic nerve head (optic disc).
The usual average optic disc diameter is about 1.5mm. A cup with a diameter
of more than 0.5mm (resulting in a cup-to-disc ratio of more than 0.3)
is generally considered to be a feature of glaucoma suspects.
| |
| Figure
2a: The pressure effect and progression of cupping |
 |
|
| Figure
2b: The progression of cupping and simultaneous deterioration
of visual field |
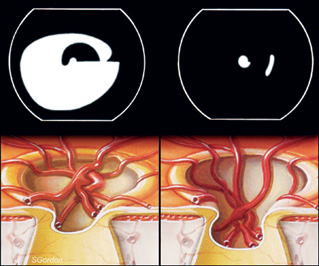 |
|
In recent years, three new techniques of optic nerve
imaging have become widely available. These are (a) scanning laser polarimetry
(GDx), (b) confocal laser ophthalmoscopy (Heidelberg Retinal Tomography
or HRT), and (c) optical coherence tomography (OCT).
The GDx machine does not actually image the optic
nerve but rather it measures the thickness of the nerve fiber layer on
the retinal surface just before the fibers pass over the optic nerve margin
to form the optic nerve. The HRT scans the retinal surface and
optic nerve with a laser. It then constructs a topographic (3-D) image
of the optic nerve including a contour outline of the optic cup. The nerve
fiber layer thickness is also measured. The OCT instrument utilizes
a technique called optical coherence tomography which creates images by
use of special beams of light. The OCT machine can create a contour map
of the optic nerve, optic cup and measure the retinal nerve fiber thickness.
Over time all three of these machines can detect loss of optic nerve fibers.
Perimetry
Perimetry is performed with either static or kinetic
presentations of the target. Kinetic perimetry (e.g., Goldmann) uses a
moving stimulus of fixed intensity. The stimulus is moved at a steady
rate from a non-seeing area to a seeing area until it is perceived by
the patient. Static perimetry (Humphrey) uses a stationary stimulus of
variable intensity. The intensity is increased until the patient first
recognizes the presence of the stimulus.
| Figure
3a: A normal Humphrey visual field |
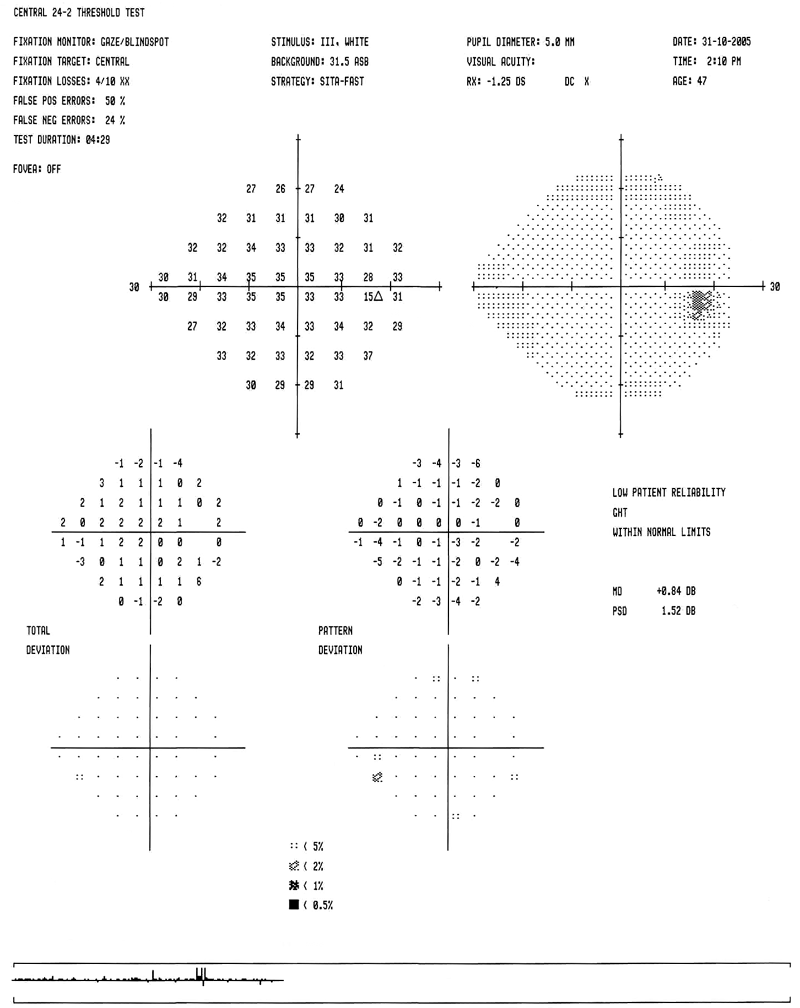 |
|
| Figure
3b: A restricted Humphrey field |
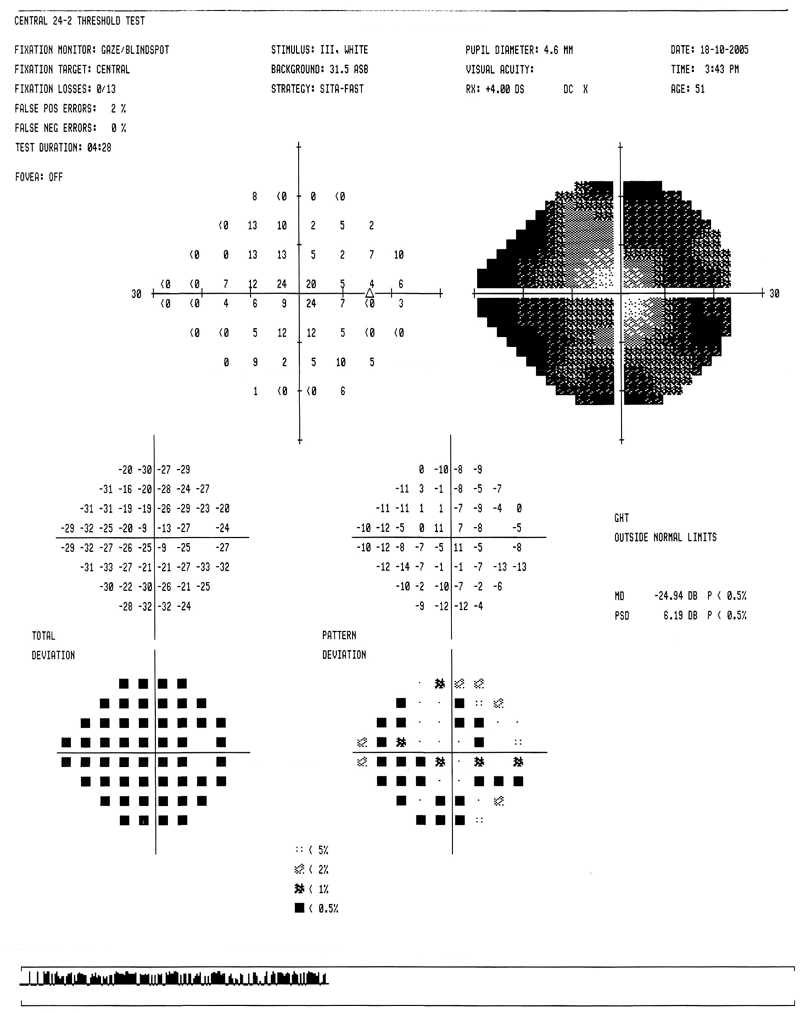 |
|
It is important to correlate changes in the visual field with
those in the optic nerve head. If an appropriate correlation is not present,
other causes of visual loss must be considered, (e.g., ischaemic optic
neuropathy, demyelinating disease, pituitary tumor, etc.)
Treatment
The objective
of glaucoma treatment is to maintain functional vision throughout the
patient's lifetime with minimal effect on quality of life. The goal of
intervention is risk factor reduction:
| |
- |
Angle control |
| |
- |
Reducing IOP |
| |
- |
Treatment of predisposing disease/factors
(diabetes mellitus, uveitis, steroids) |
Angle control
There are some situations (PACG and chronic narrow-angle
or angle-closure glaucoma) in which treatment at the angle or structures
near-by may be beneficial. Pilocarpine has the effect of contracting the
longitudinal part of the ciliary muscles and thus helps re-opening a closed
angle. In cases of acute angle-closure glaucoma or narrow-angle glaucoma,
a laser iridotomy (LI) or a laser iridoplasty also helps re-opening a
closed angle and reduces the chance of further angle-closure from happening.
Paediatric glaucomas can sometimes be controlled well with a goniotomy.
But actually it is a drainage procedure although the primary site of interest
is the angle. There are some situations in which a very swollen cataract
may push the iris forward and occlude the angle. In those cases, a simple
cataract surgery may cure the disease.
| Table
1: Mechanism of action of different drug classes |
| Mechanism of action |
Drug Class |
Preparations |
|
| Reduction of aqueous inflow |
Adrenergic agonists |
|
- Brimonidine |
| |
- Apraclonidine |
|
| Beta-blockers |
Non-selective |
| |
- Timolol |
| |
- Levobunolol |
| |
- Carteolol |
| Beta1-selective |
| |
|
- Betaxolol |
|
| Carbonic anhydrase inhibitors |
Systemic |
| |
- Acetazolamide |
| |
- Methazolamide |
| |
|
- ichlorphenamide |
| |
Topical |
| |
|
|
- Dorzolamide |
| |
|
|
- Brinzolamide |
|
| Increase in aqueous outflow
|
Cholinergics
- Increase trabecular outflow |
|
- Pilocarpine |
| |
- Carbachol |
|
Prostaglandins and other lipid
receptor agonists
- Increase uveoscleral outflow |
|
- Latanoprost |
| |
- Travoprost |
| |
|
- Bimatoprost |
| |
|
- Unoprostone |
|
Reducing IOP
Studies suggest that lowering IOP can help to slow or
stop the likelihood of disease progression.31,32 IOP can be
lowered by reducing the production of aqueous humor or increasing its
drainage "out" of the eye33-35 via (a) medical treatment, (b)
laser therapy or (c) surgery.6,7,33,35,36 Despite wide differences
in practice patterns,36 monotherapy with a topical beta-adrenergic
receptor antagonist (beta-blocker) has attained global acceptance as standard
first-line therapy for POAG.6,34,35,37-39
Unfortunately, topical beta-blockers may not be a long-term
solution. According to a multinational observational study, approximately
50% of patients with POAG or ocular hypertension required either additional
medication or a switch from beta-blocker therapy within 2 years of treatment
initiation.40 These patients with hypertension have high IOP
but there are no cupping of the disc or visual field defect. A certain
portion of them eventually develop other glaucoma features. Some ophthalmologists
regard ocular hypertension as an early state of glaucoma. Inability to
control IOP was the most frequently cited reason for treatment change.
Side effects also hampered the long-term usefulness of nonselective beta-blocker
monotherapy.
| Figure
4a: Percentage of patients requiring a change from nonselective
beta-blocker monotherapy |
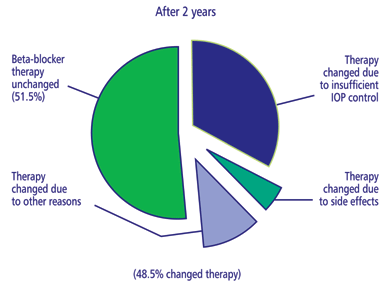 |
With the exception of the United Kingdom and the Netherlands,
where more patients undergo laser trabeculoplasty or trabeculectomy after
failure of first-line therapy, the preferred second-line therapy after
beta-blocker monotherapy is either a different monotherapy or combination
therapy. Switching to a different monotherapy is generally recommended
before resorting to combination therapy.6,39 Laser and surgical
procedures are usually used only after medical treatment has failed.2,7,33
| Figure
4b: Treatment algorithm for the management of POAG. Note that these
are only general guidelines.6 |
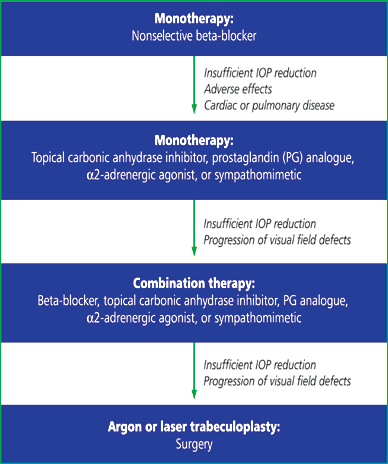 |
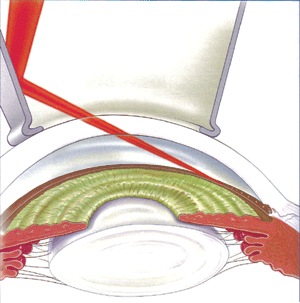
Argon laser trabeculoplasty (ALT) is a common treatment
procedure for the treatment of POAG in the West. The laser beam opens
the fluid channels (trabeculum) of the eye, helping the drainage system
to work better. In many cases, medication will still be needed. Usually,
half the trabeculum is treated first. If necessary, the other half can
be treated in a separate session another time. This method prevents over-correction
and lowers the risk of increased pressure following surgery. ALT has successfully
lowered IOP in up to 75% of patients treated.
| Figure
5a: Argon laser trabeculoplasty: laser beams are focused and reflected
to the trabeculum by mirrors in a special contact lens |
 |
Selective laser trabeculoplasty (SLT) is a new form of
trabeculum laser treatment under multi-center clinical trials. It uses
a combination of laser frequencies that allow the laser to work at very
low levels. It treats specific cells "selectively", leaving untreated
portions of the trabeculum intact. For this reason, it is believed that
SLT, unlike other types of laser surgery, may be safely repeated many
times.
Cyclophotocoaguation (YAG or Diode laser) is a "last-ditch"
procedure to save an eye from severe glaucoma damage not managed by standard
glaucoma surgery. This surgery destroys part of the ciliary body, the
part of the eye that produces intraocular fluid. This procedure may need
to be repeated in order to permanently control glaucoma.
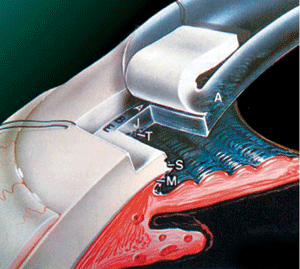
Trabeculectomy may be needed in cases in which both medical
and/or laser treatments fail to lower the IOP to the target level. In
this filtering microsurgery, a tiny drainage hole is made in the sclera
(under a pre-opened partial thickness scleral flap). If successful, the
hole will act as a fistula through which the aqueous humor flows from
the anterior chamber into the sub-conjunctival space, bypassing the highly
resistant trabecular meshwork. As a result, the IOP can be lowered to
a level at which retinal nerve fiber damage is minimized.
| Figure
5b: An opening is made at the trabeculum (T), allowing aqueous to
flow directly from the anterior chamber into the sub-conjunctival
space |
 |
In general, glaucoma filtering surgery is successful
in about 70-90% of cases. Occasionally, the surgically created drainage
fistula begins to close (wound healing) and the IOP rises again. This
occurs most often in younger patients. Anti-wound healing drugs, such
as mitomycin-C and 5-FU, help to slow down the "healing". If needed, trabeculectomy
can be done a number of times in the same eye (usually at different sites).
Glaucoma drainage devices are intraocular implants which
allow aqueous to flow from the anterior chamber into a maintained episcleral
space from where it can be absorbed into surrounding blood vessels. There
are a few commercial preparations in the market, such as Molteno, Ahmed,
Baerveldt, etc. It is indicated when there is a very high risk of failure
of trabeculectomy even with anti-fibrotics - these eyes invariably have
severe, refractory glaucoma:
| |
- |
Previously failed trabeculectomies with
anti-fibrotics. |
| |
- |
Prior multiple ocular surgeries with conjunctival
scarring. |
| |
- |
Traumatic, inflammatory or chemically induced
surface scarring. |
| |
- |
Intraocular membrane formation likely to
occlude a non-implant drainage procedure (e.g., irido-corneal endothelial
syndrome, neovascular glaucoma). |
This surgery, and the management of the patient post-operatively
is complicated. Only an ophthalmologist with appropriate training and
experience in glaucoma should perform it.
Conclusion
Life-long medical and ophthalmological follow up is
necessary with or without a good disease control. As a result of its chronic
nature, a good drug compliance and trusting doctor-patient relationship
is essential in achieving the ultimate objective:
"To maintain functional vision throughout the patient's
lifetime with minimal effect on quality of life."
Key messages
- Glaucoma is asymptomatic until in late stages during which severe
irreversible ocular damage already occurs.
- Early detection and referral of suspected cases to eye specialists
by family physicians will help preserve vision, prevent blindness and
improve quality of life.
- Risks factors include old age, family history and the female gender.
- Family physicians can help in promoting the importance of good drug
compliance, regular general and eye follow up visits and good control
of systemic factors eg., Blood pressure and sugar level.
Jackson Woo, FRCS(Glasg),
FHKAM(Ophthal)
Senior Medical Officer,
Department of Ophthalmology, Caritas Medical Centre.
Correspondence to:
Dr Jackson Woo,
Department of Ophthalmology, Caritas Medical Centre, 111 Wing Hong Street,
Sham Shui Po, Kowloon.
References
- National Advisory Eye Council. Vision Research. A National Plan:
1999-2003. Available at: http://www.nei.nih.gov/TextSite/publications/Plan/NEIExecSum/execsumm.htm.
Accessed 19 September 2001.
- Shields MB. Textbook of Glaucoma. 4th ed. Philadelphia,
PA: Williams & Wilkins; 1998:chap 1, 2, 4, 9, 27, 29.
- Flanagan JG. Glaucoma update: epidemiology and new approaches to
medical treatment. Ophthalmic Physiol Opt 1998;18:126-132.
- Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and
incidence in the United States. Invest Pohthalmol Vis Sci 1997;38:83-91.
- Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol
1996;80:389-393.
- Hoyng PFJ, van Beek LM. Pharmacological therapy for glaucoma: a review.
Drugs 2000;59:411-434.
- Fiscella RG. Costs of glaucoma medications. Am J Health Syst Pharm
1998;55:272-275.
- Zimmerman TJ, Zalta AH. Facilitating patient compliance in glaucoma
therapy. Surv Ophthalmol 1983;28(suppl):252-257.
- National Eye Institue. Facts about open-angle glaucoma. National
Eye Health Education Program. Available at: http://www.nei.nih.gov/gam/facts.htm.
Accessed 19 September 2001.
- Royal Australian and New Zealand College of Ophthalmologists. The
National Trachoma and eye Health Program, Chapter 8, pp. 82-100, Sydney,
1980.
- Baasanhu J, Johnson GJ, Burendei G, et al. Pervalence and
causes of blindness and visual impairment in Mongolia: a survey of populations
aged 40 years and older. Bull World Health Organization 1994;72:771-776.
- Foster PJ, Oen FT, Machin DS, et al. the prevalence of glaucoma
in Chinese residents of Singapore. A cross-sectional population survey
in Tanjong Pagar district. Arch Ophthalmol 2000;118:1105-1111.
- Foster PJ, Johnson GJ. Glaucoma in China: how big is the problem?
Br J Ophthalmol 2001;85:1277-1282.
- Hong Kong Hospital Authority Statistical Report 2001-2002. Registration
of permanent blindness in hospital authority ophthalmology teams 2001,
p.68. http://www.ha.org.hk
(accessed 27 Sept 2003).
- Tso MOM, Naumann GO, Zhang S. Editorial - Studies of the prevalence
of blindness in the Asia-Pacific region and the worldwide initiative
in ophthalmic education. Am J Ophthalmol 1998;126:582-585.
- Seah SKL, Foster PJ. Chew PT, et al. Incidence of acute primary
angle-closure glaucoma in Singapore. An Island-Wide Survey. Arch
Ophthalmol 1997;115:1436-1440.
- Wong TY, Foster PJ, Seah SKL, et al. Rates of hospital admissions
for primary angle closure glaucoma Chinese, Malays, and Indians in Singapore.
Br J Ophthalmol 2000;84:990-992.
- Dandona L, Dandona R, Naduvilath TJ, et al. Is current eye-care-policy
focus almost exclusively on cataract adequate to deal with blindness
in India? Lancet 1998;351:1312-1316.
- Dandona L, Dandona R, Mandal P, et al. Angle-closure glaucoma
in an urban population in southern India. The Andhra Pradesh eye disease
study. Ophthalmology 2000;107:1710-1716.
- Foster PJ, Baasanhu J, Alsbirk PH, et al. Glaucoma in Mongolia
- A population-based survey in Hovsgol Province, Northern Mongolia.
Arch Ophthalmol 1996;114:1235-1241.
- Wensor MD, McCarty CA, Stanislavsky YL, et al. The prevalence
of glaucoma in the Melbourne Visual Impairment Project. Ophthalmology
1998;105:733-739.
- Mitchell P, Smith W, Attebo W, et al. Prevalence of open-angle
glaucoma in Australia. The Blue Mountain Eye Study. Ophthalmology
1996;103:1661-1669.
- Shiose Y, Kitazawa Y, Tsukuhara S, et al. Epidemiology of
glaucoma in Japan - A nationwide glaucoma survey. Jpn J Ophthalmol
1991;35:133-135.
- Leske MC, Connell AM, Wu SY, et al. Risk factors for open
angle glaucoma. The Barbados Eye Study. Arch Ophthalmol 1995;113:918-924.
- McNaught AI, Allen JG, Healey DL, et al. Accuracy and implications
of a reported family history of glaucoma: experience from the Glaucoma
Inheritance Study in Tasmania. Arch Ophthalmol 2000;118:900-904.
- Foster PJ, Machin D, Wong TY, et al. Determinants of intraocular
pressure and its association with glaucomatous optic neuropathy in Chinese
Singaporeans: the Tanjong Pagar Study. Invest Ophthalmol Vis Sci
2003;44:3885-3891.
- Tornquist R. Shallow anterior chamber in acute angle-closure. A clinical
and genetic study. Acta Ophthalmol 1953;31(Suppl. 39):1-74.
- Okabe I, Taniguchi T, Yamamoto T, et al. Age-related changes
of the anterior chamber width. J Glaucoma 1992;1:100-107.
- Foster PJ, Alsbirk PH, Baasanhu J, et al. Anterior chamber
depth in Mongolians. Variation with age, sex and method of measurement.
Am J Ophthalmol 1997;124:53-60.
- Mitchell P, Hourihan F, Sandbach J, et al. The relationship
between glaucoma and myopia. The Blue Mountains Eye Study. Ophthalmology
1999;106:2010-1015.
- Mao LK, Stewart WC, Sjields MB. Correlation between intraocular pressure
and progressive glaucomatous damage in primary open-angle glaucoma.
Am J Ophthalmol 1991;111:51-55.
- Collaborative Normal-Tension Glaucoma Study Group. Comparison of
glaucomatous progression between untyreated patients with normal-tension
glaucoma and patients with therapeutically reduced intraocular pressures.
Am J Ophthalmol 1998;126:487-497.
- Vaughan D, Riordan-Eva O. Glaucoma. In: Vaughan D, Asbury T, Riordan-Eva
O, (eds). General Ophthalmology. 15th ed. Stamford,
CT: Appleton & Lange; 1999:200-215.
- Newell FW. Ophthalmology Principles and Concepts. 8th
ed. St. Louis, MO:Mosby-Year Book inc; 1996:379-405.
- Diestelhorst M. Medical treatment of glaucoma and the promising perspectives.
Current Opinion in Ophthalmology 1996;7:18-23.
- Jonsson B, Krieglstein G. Foreword. In: Jonsson B, Krieglstein G,
(eds). Promary Open-Angle Glaucoma: Differences in International
Treatment Patterns and Costs. Oxford, UK: Isis Medical Media Ltd;
1998:vii.
- Kobelt G, Haga A, Gerdtham U-G. Observational study. In: Jonsson
B, Krieglstein G, (eds). Primary Open-Angle Glaucoma: Differences
in International Treatment Patterns and Costs. Oxford, UK: Isis
Medical Medica Ltd;1998:17-23.
- Cantor LB. Glaucoma. In: Rakel R, (ed). Conn's Current Therapy
2000. Philadelphia, PA:WB Saunders Co; 2000:912-915.
- Camras CB, Toris CB, Tamesis RR. Efficacy and adverse effects of
medications used in the treatment of glaucoma. Drugs Aging 1999;15:377-388.
- Koblet G, Haga A, Gerdtham U-G. Results for the United States. In:
Jonsson B, Krieglstein G, (eds). Primary Open-Angle Glaucoma: Differences
in International Treatment Patterns and Costs. Oxford, UK: Isis
Medical Media Ltd; 1998:106-126.
