|
February 2007, Volume 29, No. 2
|
Update Articles
|
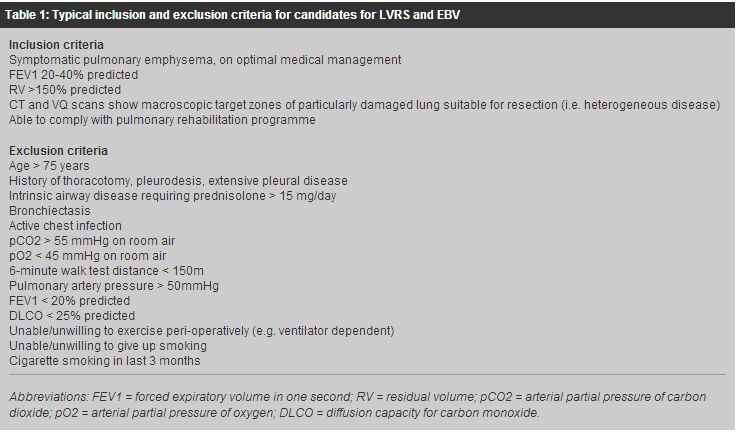
Surgical management of pulmonary emphysema: an update for primary care physiciansAlan D L Sihoe 司徒達麟, Howard H Y Chan 陳浩恩 HK Pract 2007;29:43-51 Summary Pulmonary emphysema is a major cause of chronic morbidity and mortality for which the traditional mainstay of management is medical palliation. However, advances in surgical technology have now given thoracic surgeons a major role to play in treating this disease. Lung volume reduction surgery is now proven to offer functional and survival benefit to selected patients with emphysema over medical therapy alone . The latest results from the National Emphysema Treatment Trial have conclusively demonstrated that such benefits are significant and long-lasting. More recently, endobronchial valve therapy for emphysema has been developed, promising effective functional improvements with minimal procedural morbidity. In Hong Kong, these surgical options for the treatment of emphysema are available in addition to lung transplantation. More than ever, the management of pulmonary emphysema should be regarded as a multi-disciplinary effort, involving primary care physicians and thoracic surgeons as well as respiratory and rehabilitation physicians, to ensure that every therapeutic option is made available to patients. 摘要 肺氣腫是一種常見而可致命的慢性疾病,傳統上,是以藥物進行緩解症狀治療。隨著外科醫學科技的進步,現在亦可以胸肺外科手術有效地治療肺氣腫。 與藥物治療比較肺臟容量縮減手術在某些病人組別已被證實可更有效地改善肺功能及延長壽命。美國的國家肺氣腫治療試驗計劃的最新研究結果已顯示肺臟容量縮減手術的效益是顯著和持久。 較近期,支氣管內活瓣裝置亦在研發中,希望可以用最少的手術為患者改善其肺功能。以上的各種外科療法,及肺移植手術,均可在香港進行。因此,現今的肺氣腫治療需要多方面的協力, 除呼吸系統科和康復內科醫生外,家庭醫學和胸肺外科醫生亦可參與,以確保每個病人都可以得到接受不同方式的機會。 Introduction Chronic obstructive pulmonary disease (COPD) - which includes chronic bronchitis and emphysema - remains a distressingly common disease associated with significant morbidity and mortality. In Hong Kong, an estimated 1.4% of the population is diagnosed to have COPD, and this proportion may be as high as 4.6% in those aged 65 and above.1 In 2004, COPD contributed to 5.0% of all deaths in Hong Kong and over 29300 cases of in-patient discharges and deaths.2 With the ageing of the local population, the increasing number of smokers in the Greater China region, and the worsening air pollution in the region, the impact of COPD and emphysema on the population of Hong Kong may further increase. The mainstay of emphysema management has traditionally been medical therapy aimed at symptomatic treatment, prevention of complications and improvement in quality of life. Nonetheless, medical therapy remains at best a palliative measure and cannot provide lasting correction or 'cure' of the underlying structural and physiological pathologies. In recent years, however, there has been an increasing understanding that the thoracic surgeon may have an effective role to play in the treatment of selected patients with pulmonary emphysema. The ability of surgery to remodel the respiratory anatomy may provide lasting therapy for patients with severely emphysematous lungs. This review provides an update on the surgical options for managing pulmonary emphysema now available in Hong Kong. Pathophysiology of pulmonary emphysema Pulmonary emphysema is defined as the permanent destructive enlargement of the airspaces distal to the terminal bronchioles.3 It is characterized by the loss of lung elasticity and destruction of structures supporting the alveoli. The small airways therefore collapse during expiration, so that air is trapped within the lungs and cannot be effectively expired. This gradually leads to lung hyperinflation that 'splints' the diaphragm and ribcage in an expanded state. The patient therefore finds it increasingly difficult to use the normal respiratory musculature to reduce the thoracic volume during expiration. Because emphysema is an irreversible physical process, medical therapy alone cannot correct the condition. Conventionally, the only realistic chance of such 'cure' is lung transplantation. However, transplantation itself entails significant risks of mortality and morbidity. More importantly in Hong Kong, there is a significant lack of suitable donors. The development of surgery for pulmonary emphysema As the respiratory compromise in pulmonary emphysema is due to hyperinflated lungs, a structural remodeling of the chest may help to improve the condition by reducing lung volume and restoring normal tidal excursion of the diaphragm and ribcage. Early attempts to reduce the thoracic volume at the beginning of the 20th century included chest wall surgery (costochondrectomy, thoracoplasty and phrenicectomy), use of abdominal belts, and the creation of a pneumoperitoneum.4-6 However, results were inconsistent, and concerns were raised over possible adverse effects on the respiratory function and abdominal complications. In 1957, Brantigan and Mueller proposed the direct surgical excision of parenchymal lung tissue to reduce the hyperinflated lung volume, introducing the concept of "lung volume reduction surgery (LVRS)."7 Besides reducing chest wall splinting, they also reasoned that LVRS would increase radial traction on small airways in the remaining lungs, thereby enhancing expiratory airflow. Although 75% of patients in their series had symptomatic improvement, the benefit could not be objectively documented and a high operative mortality of 18% was observed. This prevented widespread acceptance of the procedure, and the idea of LVRS was left dormant for the next 30-odd years. In 1993, Dr Joel Cooper became convinced that with modern anaesthesia and better peri-operative care, the concept of LVRS was feasible, and began modifying Brantigan's original approach.8 He introduced a vigorous regime of pre-operative pulmonary training, and used a median sternotomy approach to resect 20% to 30% of the most diseased portions of the lungs in 20 emphysematous patients. No operative mortality was reported. All patients had significant improvements in forced expiratory volume in one second (FEV1) and six minute walk test (6MWT) results. Moreover, many patients no longer required supplemental oxygen after surgery. This demonstration that LVRS could be safely performed rekindled interest in the surgical management of pulmonary emphysema. Subsequent studies demonstrated the convincing physiological basis for respiratory improvement after LVRS. Sciurba and colleagues9 found that elastic recoil in the lungs increased 38% after LVRS. Patients who had greatest gains in elastic recoil also had the greatest increases in 6MWT distance and in reductions of residual volumes. It was shown in other studies that objective measurements of lung hyperinflation, respiratory muscle function, and even pulmonary hypertension could be reversed by LVRS.10,11 Since Cooper's initial report, more than 30 series reporting over 2000 operations have been published,12-15 confirming that patients experienced clinical improvement in lung function, exercise tolerance and quality of life at up to 2 to 3 years following LVRS. Mortality rates were typically between 3% and 9%. Nevertheless, the patient numbers in these early studies were small, and most of these reports were limited by heterogenic patient cohorts, wide variations in the surgical techniques used, lack of standardized outcome measures, and lack of long term follow-up. Crucially, most of these early studies lacked comparison with a control group. National emphysema treatment trial To convincingly establish the efficacy of LVRS, the first major multi-centre, randomized controlled trial on the surgical management of pulmonary emphysema was commenced in the late 1990s in the United States. The National Emphysema Treatment Trial (NETT) recruited 1218 patients with pulmonary emphysema from 17 different medical centres between January 1998 and July 2002.16 After completion of a 6-10 week rehabilitation programme, patients were randomly assigned to either continue medical treatment only (n = 610) or to have LVRS in addition to continued medical treatment (n = 608). The first interim report from the NETT in 2001 suggested that overall mortality rate at 30 days after commencement of treatment was significantly higher in the LVRS group than in the medical therapy group.17 However, subgroup analysis showed that the high mortality in the LVRS group was mainly accounted for by a specific high-risk subset of patients who had a forced expiratory volume in one second (FEV1) no more than 20% of their predicted value, and had either a homogeneous distribution of emphysema on computed tomography (CT) or a carbon monoxide diffusion capacity (DLCO) that was no more than 20% of their predicted value. After excluding these high-risk patients, the mortality rates for the remaining 1048 participants were actually similar in the LVRS and medical treatment only groups throughout follow-up. From May 2001, NETT no longer recruited patients meeting the high-risk criteria. These criteria are now broadly taken as contra-indications for LVRS. The results of NETT were ultimately published in the New England Journal of Medicine in May 2003.16 After 24 months of follow-up, exercise capacity had improved by more than 10 watts on bicycle testing in 15% of the patients who received LVRS, as compared to 3% of patients in the medical-therapy group (P<0.001). Overall, more patients in the LVRS group also improved in terms of spirometry results, symptoms, 6MWT and dyspnea scores, and quality of life than in the medical group. These improvements gradually declined over 24 months to pre-operative levels. However, during the same period, these same outcome measures had worsened to below their pre-treatment levels in patients who received medical treatment alone. Cost-effectiveness analysis showed that LVRS was less cost effective in the first year following surgery due to procedure-related expenses and postoperative inpatient care.18 However, the difference in total medical care costs between the LVRS and medical treatment only groups had shrunken considerably by the third year of follow up. Two predictors of benefit following LVRS were noted: the predominance of emphysema in either the upper or the non-upper lobes on CT scan, and the pre-treatment exercise capacity. Patients with mostly upper-lobe emphysema and low exercise capacity were significantly more likely to live longer and to have better functional outcomes after LVRS than after medical treatment. The cost-effectiveness estimates were also most favourable for LVRS in these patients. Those with mostly upper-lobe emphysema but high exercise capacity enjoyed measurable functional (but not survival) benefits after LVRS compared to medical therapy alone. Patients with mostly non upper-lobe emphysema and low exercise capacity had symptomatic benefits after LVRS. Patients with mostly non upper-lobe emphysema and high exercise capacity had poorer survival after LVRS than after medical treatment. After 24 months of follow-up, amongst patients with predominantly upper-lobe emphysema and low exercise capacity, mortality after LVRS was less than 50 percent of that after medical therapy alone (risk ratio for death = 0.47; P=0.005). Among patients with non-upper-lobe emphysema and high exercise capacity, mortality was twice as high in the LVRS group as in the medical therapy group (risk ratio = 2.06; P=0.02). The longer-term survival benefit with LVRS was further confirmed in the latest follow-up results from NETT published in August 2006.19 After a mean of 4.3 years, the intention-to-treat analysis demonstrated a significant survival advantage for LVRS, with a 5-year risk ratio for death of 0.86 compared to medical therapy alone (p = 0.02). Maximal exercise capacities were better in the LVRS group through 3 years of follow-up, and health-related quality of life to be better through 4 years. This 2006 report also reconfirmed that for patients with upper-lobe emphysema and low exercise capacity, improved survival through 5 years (RR, 0.67; p = 0.003), exercise capacity through 3 years (p < 0.001), and symptoms through 5 years (p < 0.001 years 1 to 3, p = 0.01 year 5) could all be demonstrated after LVRS. Even for patients with upper-lobe predominance but high exercise capacity, LVRS could improve exercise capacity through 3 years (p < 0.01) and symptoms through 4 years (p < 0.01), although survival was not affected significantly. Patient selection and workup for LVRS These results from NETT provide conclusive evidence that LVRS can therefore confer significant and lasting benefits for patients with emphysema. However, cardiac and pulmonary morbidities after LVRS are reported in 20% and 29.8% of patients respectively, and it is clear that certain subgroups of patients may be at higher risk for LVRS than others.20 Thus careful patient selection is imperative. The patient selection criteria now generally adopted by most surgical units are summarized in Table 1. In general, patients with severe emphysema who have debilitating symptoms not responding to maximal medical treatment may be considered for surgical management. It is particularly important to identify patients with emphysema-predominant COPD as opposed to those who may have bronchiectasis-, bronchitis- or asthma-predominant disease in whom LVRS will not correct the underlying pathophysiology. The basic workup for potential surgical candidates include arterial blood gas analysis, CT scanning of the thorax, ventilation-perfusion (VQ) scanning of the thorax, lung spirometry, lung volume analysis, DLCO testing, and exercise capacity testing (such as the 6MWT). Investigation and optimization of all medical therapy for any co-morbidities is a pre-requisite.
The first step is to exclude those patients defined as high-risk according to NETT findings. An FEV1 less than 20% predicted plus a DLCO less than 20% predicted and/or homogeneous distribution of disease on CT or VQ scanning are now generally taken to be absolute contra-indications for LVRS. Hypercapnia and pulmonary hypertension are also considered strong contra-indications. Patients with heterogeneous disease on pre-operative CT or VQ scanning - with particularly diseased zones of the lung(s) targeted for resection - typically benefit most from LVRS. Among these patients, those with low exercise capacity have the most survival and functional advantage, but even those with higher exercise capacity benefit functionally from LVRS. Where possible, patients should be enrolled to a programme of pulmonary rehabilitation before surgery, which includes education, counselling, exercise training, and physiotherapy to optimize their pre-operative functional capacity. This training should ideally be for 6 to 10 weeks in order to maximize post-operative recovery potential. An effective pulmonary programme is reliant on a multi-disciplinary effort, including the primary care physician as well as hospital respiratory specialists and physiotherapists. Surgery for pulmonary emphysema: current practice in Hong Kong The only real 'cure' of the lung destruction process underlying emphysema is lung transplantation. In Hong Kong, the Grantham Hospital is the only centre performing this complex surgery. Since the first lung transplantation in Hong Kong in 1995, 11 cases of lung or heart-lung transplantations have been performed at the Grantham hospital. Two double lung transplantations were performed for patients with severe pulmonary emphysema. One has died 40 months after transplantation, but the other is alive and well at the time of this writing at over 65 months after transplantation. Nonetheless, the number of transplants possible in Hong Kong is limited by a serious lack of suitable organ donors. Therefore, LVRS remains the mainstay surgical treatment for the majority of patients with severe pulmonary emphysema in Hong Kong. When performing a typical LVRS, the thoracic surgeon aims at the removal of 20-30% of the total lung volume to correct the hyperinflation underlying severe emphysema. Often no single discrete emphysematous lung bulla can be demarcated or targeted for bullectomy. The results of LVRS are therefore especially dependent on the expertise and judgment of the specialist thoracic surgeon. There is some debate over whether unilateral or bilateral LVRS should be performed. Bilateral LVRS is sometimes suggested to result in higher mortality rates and longer hospital stays,21 but is reported to give significantly better functional improvement and better 2 year survival than unilateral LVRS.21-23 Unilateral LVRS can be considered if one particular chest side is relatively contraindicated for surgery, such as in cases of previous tuberculosis infection, pleurodesis or thoracotomy. The conventional surgical approach for bilateral LVRS is through a median sternotomy. Unilateral LVRS can be performed via an open thoracotomy. Both of these have large, relatively painful wounds. However, an alternative is the video-assisted thoracic surgery (VATS) approach. This minimally invasive surgical technique typically uses only three 10mm wounds on each side of the chest, and is proven to give equal effectiveness but significantly less pain and morbidity than conventional open surgery.24-25 In the NETT study, patients operated on at centres that performed both median sternotomy and bilateral VATS approaches were randomly assigned to receive one of these approaches for LVRS. No difference between the groups was seen in terms of functional or physiological outcomes and post-operative morbidity and mortality rates.26 However, with the VATS approach, post-operative lengths of hospital stays were shorter and overall treatment costs were also lower. Other studies have shown that the VATS approach for LVRS gives significantly reduced incidences of respiratory failure and total in-hospital mortality.21 Which surgical approach used by the surgeon may therefore be taken into consideration by the primary care physician referring a patient for LVRS. Regardless of the approach used, the overall mortality risk during the first three months after LVRS is 5.2% after the high-risk patients have been excluded according to NETT results.20 Incidences of major cardiac and pulmonary complications are typically around 20% and 30% respectively. Major cardiac complications include myocardial infarction and arrhythmia, while major pulmonary complications include prolonged ventilation, pneumonia and re-operation for persistent air-leak. The most common post-operative complication is persistent air leak with a reported incidence of up to 45%.27 The most common surgical technique to reduce this is buttressing of the staple line with bovine pericardium strips.28 In Hong Kong, both the VATS and median sternotomy approaches are used. Mechanical stapling with pericardial buttresses for resection of the lung is routinely performed. It is the authors' preference to perform bilateral VATS where possible. For patients at high-risk for post-operative complications, a 2-stage approach for bilateral surgery (operating first on one side then the other in a separate operation) is sometimes safer. It is the authors' practice to observe all patients in the Intensive Care Unit (ICU) or High-Dependency Unit (HDU) initially after surgery. All patients are mobilized and a vigorous physiotherapy is commenced as soon as possible after surgery. A multi-disciplinary programme of post-operative recovery and rehabilitation is employed, and is jointly managed by the thoracic surgical team and the referring respiratory medical team. Barring major complications, many patients can be discharged home within a few weeks or less after surgery. Subsequent reinforcement of the rehabilitation programme and optimization of medical therapy relies on close co-operation with primary care physicians. Endobronchial therapy for emphysema Despite the benefits of LVRS, this treatment remains a major surgical operation performed on sick patients. To address patients who may not be able to tolerate major surgery, a novel approach has recently emerged: endobronchial therapy. If the lung volume can be reduced through bronchoscopic interventions, the patient may potentially reap the benefits of LVRS without having to undergo major resection surgery. Several endobronchial approaches have been described to cause collapse of specific diseased areas of the lung to achieve lung volume reduction. These include endobronchial valve implants, bronchial fenestration, and occlusion of the airways with umbrella blockers or injection of fibrin glue.29,30 Bronchial fenestration uses radiofrequency or mechanical penetration of the bronchial wall plus stent placement into the created fistula to allow the entrapped air in a hyperinflated part of the lung to drain out to a patent airway. Initial studies have demonstrated that bronchial fenestrations can improve expiratory flow in emphysematous human lungs.29,31 However, stent restenosis remains the major problem. The most studied approach is the placement of endobronchial valve (EBV) implants.32 These implants are silicone-based, one-way valves mounted on a nitinol frame (Figure 1). These are placed to the lobar, segmental or sub-segmental level bronchi. Placement is typically performed in the operating theatre using rigid bronchoscopy with the patient heavily sedated but spontaneously breathing. The EBV will allow venting of air and secretions from the parts of the lung distal to valve whilst preventing air from entering. The result is collapse of the lung distal to the site of EBV placement, effecting a reduction in lung volume.
The most studied design of EBV has been the Emphasys EMB (Emphasys Medical; Redwood City, CA) 33-36. The largest single series in the world using this EBV was performed in Hong Kong, and had been reported by the author (ADLS) and co-workers in 2004.35 We reported a series of 21 patients with incapacitating emphysema who underwent EBV placement. There was no mortality and the mean hospital stay was 5.60 6.37 days. Four patients developed pneumothorax. Compared with pre-treatment levels, FEV1 and mean forced vital capacity improved significantly at 90 days. The mean 6MWT distance also significantly improved at 30 and 90 days. The results of the 36-Item Short-Form Health Survey and the St George Respiratory Questionnaire showed significant improvements in quality of life at 90 days. The Medical Research Council dyspnea scores also improved significantly at 30 and at 90 days. More recently, a systematic review of all the published series using this design of EBV (including our series) was undertaken.37 A total of 396 EBV implants were placed in 98 patients in 7 countries. Eight patients (8.2%) had serious complications in the first 90 days, including four patients with prolonged air leak for over 7 days, three cases of pneumothorax requiring surgical intervention, and one mortality (1.0%) resulting from pneumonia. However, significant lung function and exercise capacity improvements were demonstrated. FEV1 increased by 10.7 +26.2% (p = 0.007), FVC increased by 9.0 +23.9% (p = 0.024), and 6-min walk distance increased by 23.0 +55.3% (p = 0.001). There was also a trend towards improvement in DLCO (17.2 +52.0%, p = 0.063). Two predictors of better results were noted. Firstly, patients treated unilaterally showed a trend toward greater improvement than those treated bilaterally. Secondly, a trend toward more improvement was observed in patients who received complete lobar exclusion with EBV implants compared to those with just one or two bronchopulmonary segments treated. Subsequent to this systematic review, other series have continued to report lasting improvements in pulmonary function and exercise testing after EBV placement.38 Although it has been hypothesized that the EBVs can be covered by subsequent granulation tissue overgrowing the implants, this has not noticeably affected mucus draining through the EBVs thus far.38 Rates of post-obstructive pneumonia developing in the lung segments excluded by EBV implants have been remarkably low.37 In the rare instances where an in situ EBV implant is suspected to be causing clinical problems, the EBV can also be readily removed by fibre-optic bronchoscopy.35,37 The placement of EBV implants should be performed by specialist thoracic surgeons for several important reasons. Firstly, in patients with poor lung function, extended instrumentation in the airways is potentially hazardous, not to mention is highly uncomfortable for the patient. Use of a rigid ventilating bronchoscope for placement by the surgeon allows both good control of the proximal airway and deep sedation of the patient throughout the procedure. The procedure should therefore be performed in an operating theatre with an experienced anaesthetist present who can promptly intubate the patient if required. Secondly, the most commonly observed major complication thus far reported is of air-leakage. Around half of those patients developing post-EBV placement pneumothorax will require specialist surgical treatment. Thirdly, patients with emphysema generally have a higher risk for lung cancer. Pulmonary nodules visualized at the time of EBV placement or subsequently may therefore pose clinical dilemmas.39 A nodule located in the lobe targeted for collapse will be difficult to identify after EBV implantation. Even for a nodule appearing in the non-collapsed parenchyma, distortion of the pulmonary anatomy after EBV placement can make surgical localization more difficult. Conversely, placement of EBVs before lung resection for a pulmonary nodule may improve pulmonary function in patients with severe emphysema, thereby reducing the risk of surgery. These decisions must be made by the thoracic surgery specialist and will dictate the sequence of EBV placement and lung resection. Fourthly, should the EBV approach fail to benefit the patient, the patient should have recourse to LVRS or even lung transplantation. Comprehensive management and balanced counselling for the patient should ideally always involve the thoracic surgeon who has all these approaches at his/her disposal. Since 2004, EBV implantation has become available in Hong Kong to patients with severe emphysema. As the use of EBV therapy is still in its infancy, the current selection criteria are the same as for LVRS (Table 1). Primary care physicians who feel that their patients may benefit from EBV therapy can refer to the LVRS selection criteria and discuss individual cases with the thoracic surgeon. However, it is anticipated that thresholds for EBV therapy may gradually be lowered with increasing experience. Pre-operative pulmonary rehabilitation is given for all patients, and post-operative multi-disciplinary pulmonary rehabilitation mandatory. Admission to ICU or HDU is required only in a minority of patients with respiratory complications immediately after EBV placement. It is the authors' standard practice to offer complete lobar exclusion of the one lobe with maximum disease. Unilateral EBV placement is the norm, although subsequent contralateral placement can be offered in selected patients as appropriate. Following discharge, surveillance bronchoscopy on a weekly or bi-weekly basis for the first 1-2 months is usually offered to reconfirm valve position and function. Currently in Hong Kong, each EBV implant costs in the region of HK$20,000-30,000, and a complete lobar exclusion will typically require 2-4 EBV implants. Conclusion Advances in surgical technique and technology now means that the management of pulmonary emphysema is no longer solely reliant on medical therapy. Increasingly, thoracic surgery can play a safe and effective role in the palliation of severe emphysema in selected patients. Recent evidence - notably from the NETT results - has proven that LVRS can effect long term benefits in terms of survival, exercise tolerance and quality of life in such patients. The keys to successful surgical outcomes are careful patient selection and a multi-disciplinary approach involving primary care physicians, respiratory physicians, and physiotherapists as well as specialist thoracic surgeons. In Hong Kong, a lung transplant programme is available for patients with advanced pulmonary emphysema. However, the numbers treated are limited by a lack of suitable donors. In such situations, LVRS is proven to be an effective alternative or bridge to lung transplantation in patients with emphysema.40 Fortunately, LVRS has become well established now in Hong Kong, and can be performed effectively using a minimally invasive VATS approach. Thoracic surgeons in Hong Kong have also pioneered the use of EBV implantation for the treatment of pulmonary emphysema. Primary care physicians in Hong Kong can now offer selected patients with this disease a range of surgical therapeutic options in addition to traditional medical treatments. Key messages
Alan D L Sihoe, MBBChir(Cantab), FRCSEd(CTh), FCSHK, FHKAM(Surgery)
Associate Consultant, Howard H Y Chan, MBChB(CUHK), MRCSEd Resident, Cardiothoracic Surgery Unit, Grantham Hospital. Correspondence to : Dr Alan D L Sihoe, Division of Cardiothoracic Surgery,Department of Surgery, The University of Hong Kong,Grantham Hospital, Hong Kong.
References
|
|