|
June 2014, Volume 36, No. 2
|
Update Article
|
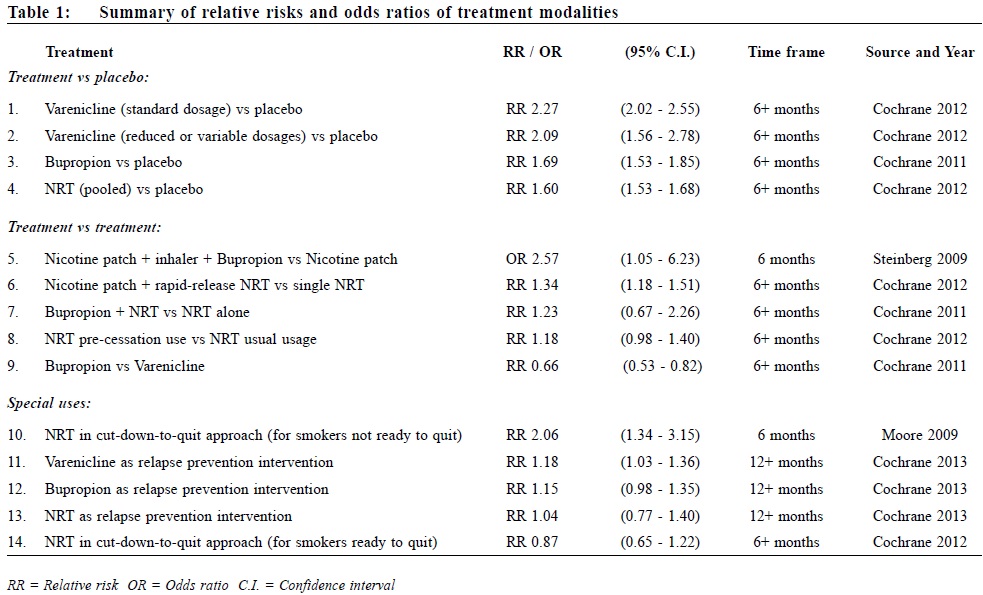
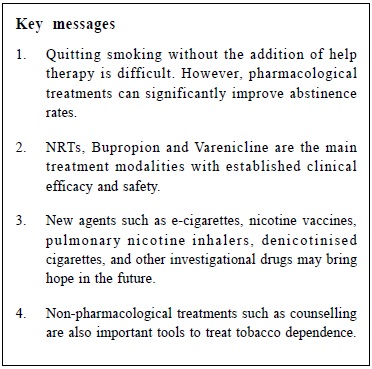
Review on pharmacological treatments for smoking cessationLing Lo 盧令, Tseng-kwong Wong 黃增光, David VK Chao 周偉強 HK Pract 2014;36:52-60 Summary Currently available effective treatments for nicotine dependence help smokers to quit more successfully than quitting without assistance. Researches on new medications and treatment modalities are rapidly growing. This ar t icle aims to review the current evidence of commonly used treatments such as nicotine replacement therapy (NRT), Bupropion, Varenicline, and to update the development of novel agents like Rimonabant and Taranabant, nicotine vaccines, pulmonary nicotine inhalers, e-cigarettes, denicotinised cigarettes, and some other investigational drugs. Pharmacogenetics in relation to smoking cessation is also discussed. 摘要 現有的有效戒煙治療能幫助吸煙者比獨自戒煙更成功地戒除對尼古丁的依賴。新的藥物和治療方式的研究正在迅速增長。本文一方面旨在檢討現行常用的治療方法,如尼古丁替代療法,安非他酮,伐尼克蘭之最新的醫學實證,另一方面更新各新療法如Rimonabant,Taranabant,尼古丁疫苗,尼古丁肺部吸入劑,電子香煙,脫尼古丁香煙,以及其他一些研究藥物的最新發展。本文也討論了一些與戒煙有關的基因藥理學。 Introduction Since the United States Public Health Service (USPHS) released its influential 2008 guideline on smoking cessation1 5 years ago, confirming the efficacy of nicotine replacement therapies (NRTs), Bupropion, and Varenicline, what breakthrough have we seen in smoking cessation research? In this review, focus is placed on a current update of some recent controversies on drug safety and a discussion of the efficacy of several emerging new agents (footnoteò). Nicotine Replacement Therapies Nicotine Replacement Therapies (NRTs) are among the oldest and the most widely studied treatment modalities in modern day smoking cessation. Studies of people attempting to quit on their own suggest that success rates after 6 to 12 months are 3 to 5%.2 The latest Cochrane review3 ascertained the pooled efficacy of NRT with risk ratio (RR) of six months abstinence at 1.60 (95% confidence interval [CI] 1.53 to 1.68) when compared with placebo. The chances of stopping smoking were increased by 50-70%. These results were observed when NRT was used as monotherapy with standard treatment duration. Recent studies have examined the use of NRT in the following aspects:
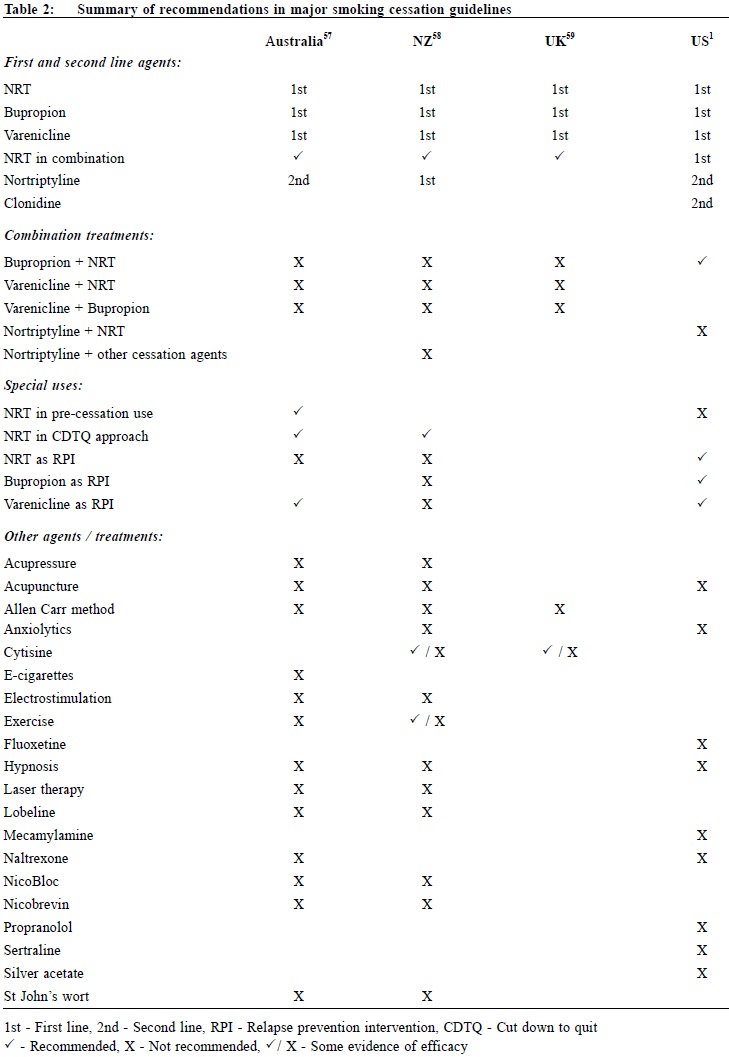
Although Clonidine and Nortriptyline are recommended by the 2008 USPHS Guideline as second-line agents, they are not discussed in this article as they have not been approved for smoking cessation in most countries, including the United States (US) and the United Kingdom (UK), mainly because of their adverse event profiles. Combination NRTs The rationale of concurrent combination NRT centres on the fact that different forms of NRTs release nicotine at different rates to the body. Nicotine patch provides steady nicotine round-the-clock, whereas nicotine gum, lozenges, nasal spray, and inhaler provide extra nicotine on demand in a more readily absorbed form (footnote #). Basal nicotine prevents withdrawal symptoms while ad libitum NRT releases nicotine to deal with sudden cravings. (This is analogous to the basal plus prandial use of insulin in diabetes treatment). The latest Cochrane review3 reported that combining a nicotine patch with a rapid-delivery form of NRT was more effective than a single type of NRT (RR 1.34, 95% CI 1.18 to 1.51 at 6+ months). A recent randomised controlled trial (RCT) compared a triple combination of nicotine patch, nicotine inhaler, and Bupropion with nicotine patch alone among smokers with comorbid medical illnesses.4 The triple combination therapy has higher 6-month abstinence rates with an odds ratio (OR) of 2.57 (95% CI 1.05 to 6.32). This response is also superior to Bupropion and single NRT combination (see below). NRT as relapse prevention intervention In 2010, a systematic review5 found that NRT appeared to be effective in preventing relapse at medium-term (6-9 months) (OR 1.56, 95% CI 1.16 to 2.11) and long-term (12-18 months) (OR 1.33, 95% CI 1.08 to 1.63). In the same year, a study in the UK confirmed that NRT as relapse prevention intervention (RPI) is also cost-effective for the National Health Service (NHS) Stop Smoking Services (SSS).6 However, the latest Cochrane review7 in 2013 failed to detect an effect of NRT in preventing relapse at 12+ months (RR 1.04, 95% CI 0.77 to 1.40). The failure was attributed to low compliance in the trials reviewed, and that one study changed regime during the relapse prevention period. The disagreement of the 2013 Cochrane review and the 2010 review was explainable by subgroup decisions. The Cochrane review suggested that NRT could be effective in unaided abstainers but did not detect an effect in assisted abstainers, while the 2010 review merged the two groups and reported a significant effect overall. Further studies are warranted to establish its cost-effectiveness and feasibility in other smoking cessation centres and perhaps in over-the-counter settings. Continual use of NRT in relapse The approved product labels for NRTs explicitly advise against the use of NRT while smoking. Most health care providers recommend discontinuation of NRT during a relapse, with concerns about adverse effects from concomitant use. A recent review of persistent NRT use, including the concomitant use of NRT while smoking, showed that this practice poses little to no risk.8 Furthermore the use of NRT in relapse does not cause harm and contrarily, may enhance the chance for eventual tobacco abstinence. Experts from the Mayo Clinic in the US recommend against stopping NRT among smokers who experience a relapse. Patients who experience nausea, which may be an indication of excessive nicotine, should be advised to reduce the NRT dose.9 Pre-cessation use of NRT (NRT Pre-loading) In the past, NRT should be initiated on the day of quitting, in contrast to the pre-cessation use of Bupropion or Varenicline. Pre-loading with nicotine patch 2 weeks prior to quit date can double abstinence rates (22% vs 11%) at 10 weeks, particularly in smokers with low nicotine dependence.10 On the other hand, 4 weeks pre-loading with nicotine gum did not improve abstinence rate (20.8% vs 19.4%) at 12 months.11 In the 2012 Cochrane review3 the RR of quitting for NRT used for a short period prior to the quit date was 1.18 (95% CI 0.98 to 1.40) at 6+ months, just missing statistical significance, though the efficacy increased when only patch trials were pooled and when one trial in which there was likely to have confounding finding was removed. NRT assisted smoking reduction Nicotine assisted reduction to stop, is also termed cut down then stop, cut down to stop, cut down to quit, or reduce to quit. This refers to reducing tobacco consumption without setting a specific quit date. Controversies exist as whether this approach is efficacious or cost effective. For smokers who are not ready to quit, this approach with behavioural support may be more effective than brief advice alone. In a local RCT of smoking-reduction counselling plus NRT intervention for smokers not willing to quit, the intervention group achieved higher 6-month abstinence and smoking-reduction rates (abstinence rates 17.0% vs 10.2%, P=0.01; and reduction rates 50.9% vs 25.7%, P=0.001).12 The study suggested that smoking-reduction aided by NRT ultimately led to more successful quit attempts. These results are supported by a meta-analysis of NRT for smokers not ready to quit13, where the 6-month abstinence rates were low but statistically significantly higher in subjects receiving NRT compared with controls (6.75% vs 3.28%, RR 2.06, 95% CI 1.34 to 3.15). A metaanalysis in the UK showed that this approach is highly cost effective compared with no quit attempt in various economic assumptions despite a low absolute quit rate at 12 months (5.3% with NRT vs 2.6% with placebo).14 For smokers who are ready to quit, however, NRT assisted smoking-reduction appears to be ineffective. In a recent RCT of 746 smokers who wanted to quit gradually and were given nicotine lozenges several weeks prior to the quit date, subjects randomised to gradual cessation were less likely to make a quit attempt than those in the abrupt cessation group (48% vs 64%) after 6 months.15 In the gradual quit group, every week of delay to the quit date increased the probability of reverting to smoking by 19%. The latest Cochrane review indicated that gradual reduction offered no advantage over abrupt quitting (RR 0.87, 95% CI 0.65 to 1.22) at 6+ months.16 Bupropion Originally developed as an anti-depressant, Bupropion in sustained release form was approved for smoking cessation in the US in 1997. The latest Cochrane systematic review17 affirmed its efficacy (RR 1.69, 95% CI 1.53 to 1.85 at 6+ months). From available data, Bupropion appears to be equally effective and of similar efficacy to NRT. However, the addition of Bupropion to NRT does not provide additional long-term benefit (RR 1.23, 95% CI 0.67 to 2.26, at 6+ months in 6 trials). Bupropion was shown to have a lower abstinence rate than Varenicline (RR 0.66, 95% CI 0.53 to 0.82 at 6+ months). It is also associated with a 1 in 1000 risk of seizures. Bupropion has also been associated with suicide risk, but whether this is causal is unclear. In terms of preventing relapse, Bupropion narrowly missed a significant effect at 12+ months (RR 1.15, 95% CI 0.98 to 1.35).7 Varenicline Varenicline is a partial agonist at the nicotine acetylcholine receptor (nAChR) specifically developed as an anti-smoking medication. It received a fasttrack approval in the US in 2006. In the latest 2012 Cochrane review18, Varenicline demonstrated a RR of 2.27 (95% CI 2.02 to 2.55) for continuous or sustained abstinence at 6 months or longer at standard dosage versus placebo. Varenicline at lower or variable doses was also shown to be effective, with an RR of 2.09 (95% CI 1.56 to 2.78) at 6+ months. Its clinical efficacy was affirmed by another meta-analysis in the same year.19 To date, one study investigated the efficacy of extended Varenicline use in relapse prevention20 in 2006. Smokers were offered 12+12 weeks of Varenicline and followed up to 52 weeks. 63% of those quit after 12 weeks had relapsed by the end of one year, compared to only 56% of those who had a further 12 weeks of therapy. A Cochrane review18 in 2012 commented that patients who took Varenicline had higher relapse rates than those on placebo during the non-treatment period, and that longer follow-up than 52 weeks is warranted to delineate the eventual relapse trajectory of extended Varenicline therapy. Another Cochrane review7 in 2013, however, affirmed in principle its efficacy in reducing relapse. Varenicline in combination therapies is not well published in the literature. Varenicline combination therapy with NRT was shown to be well-tolerated despite worries that the combo might induce excessive nausea.21 However, the trial did not demonstrate superior effect on 6-month abstinence. The combination of Varenicline and Bupropion was shown in one phase II clinical trial of 38 smokers to yield an abstinence rate of nearly 60% after 6 months.22 A larger randomised double-blind trial to investigate this hypothesis completed in April 2013 with results to be published (ClinicalTrials.gov Identifier: NCT00935818). Varenicline and increased cardiovascular risks In June 2011, the US Food and Drug Administration (FDA) issued a warning to prescribers that Varenicline may be associated with certain serious cardiovascular adverse events, basing on data from an RCT of smokers with known cardiovascular disease (CVD).23 In the same year, a meta-analysis24 of 8,216 participants in 14 RCTs demonstrated a small but statistically significant increase in serious cardiovascular adverse events in subjects receiving Varenicline compared with placebo after one year (Varenicline 1.06% vs placebo 0.82%; OR 1.72, 95% CI 1.09 to 2.71). All-cause mortality was similarly low in both groups (7 of 4908 in Varenicline subjects vs 7 of 3308 in placebo subjects). Two major limitations of the study were that the adverse events were unadjudicated and that the attrition rate in the placebo arm was higher in most of the studies included in the analysis. These introduced bias in determining serious adverse cardiovascular events that favoured fewer events counted in the placebo group. In 2012, another systematic review and meta-analysis25 of 22 trials concluded differently that Varenicline was not associated with excessive CVD risks. The CVD serious adverse events were found to be 0.63% (34/5431) in the Varenicline groups and 0.47% (18/3801) in the placebo groups. The risk difference of 0.27% was neither clinically or statistically significant. These confirmatory findings were derived from a study design where only events occurring within and shortly after treatment periods were counted. Overall, the low absolute increase in CVD risk was outweighed by the large benefit from smokingcessation with subsequent CVD risk reduction. It is therefore suggested that Varenicline should continue to be used to treat smokers with stable CVD while waiting for more definitive researches. Varenicline and neuropsychiatric symptoms and depression In February 2008, the US FDA issued an advisory reporting a possible association between Varenicline and an increased risk of behavioural change, agitation, depressed mood, and suicidal ideation and behaviour. Then in 2009, a post-marketing analysis of 80, 660 smokers attempting to quit revealed similar rates of self-harm, depression, and suicidal thoughts for both Varenicline and NRT.26 In the same year, a review in the UK27 and another pooled analysis of 12 RCTs failed to find an association between Varenicline and multiple neuropsychiatric symptoms.28 However, there was a modest association between depressed mood (RR 1.42, 95% CI 0.96 to 2.08) and Varenicline at 12 months. Overall, the RR of combined serious adverse events from Varenicline compared with placebo is estimated to be 1.36 (95% CI 1.04 to 1.79).18 However, this is based on simple counts across the trials of participants reporting one or more such events. It does not distinguish between events attributed to and those unrelated to treatment, or between those occurring during the medication and the follow-up phases. Rimonabant and Taranabant Rimonabant was first developed as an anti-obesity agent in 2006. It is a central cannabinoid-1 (CB1) receptor antagonist which is implicated in brain reward function, and is thought to have a role in controlling food consumption and in dependence and habituation. It received initial enthusiastic expectation as it targeted both nicotine dependence and the weight gain concern of potential quitters. Although Rimonabant29 is effective in smoking cessation, both the FDA and Europe disapproved its use in 2007 and 2008 because of a high incidence of depression and suicide. Another CB1 antagonist, Taranabant, was also suspended by the drug company because of unacceptable adverse events.29,30 Nicotine vaccines Vaccination against nicotine dependence is perhaps one of the most stimulating areas in the smoking-cessation pipeline. Nicotine vaccines work by inducing the immune system to produce antibodies to bind to circulating nicotine and preventing it from crossing the blood-brain barrier. None has been proven to result in statistically significant difference in long-term cessation rates.31 Nevertheless, post hoc analysis showed that smokers with higher antibodies level had higher abstinence rates. Perhaps vaccines with enhanced immunogenicity may have greater potentials. One clinical trial is now in progress to investigate the efficacy and safety of nicotine vaccine co-administered with Varenicline.32 Pulmonary nicotine inhalers Oral nicotine inhalers are inferior in pharmacokinetics to cigarette smoking, limiting its efficacy as a cessation tool. Several pulmonary inhalation systems have been developed to target nicotine to the lung. These inhalation systems range from nebulisers, metered-dose inhalers, novel pulmonary devices, to electronic cigarettes.33 Despite its theoretical advantage, pulmonary nicotine inhalers face regulatory (for instance its addictive potential) and technical (for example particle size) barriers to their development. Electronic cigarettes are more promising and will be discussed below. E-cigarettes Electronic cigarettes (e-cigarettes) are formally termed by the World Health Organisation as Electronic Nicotine Delivery Systems (ENDS). They are cigarette-like battery-powered devices that vapourise nicotine from a cartridge that can be inhaled. They address both the nicotine dependence and psychological dependence on cigarettes as the source of nicotine. The vapour from e-cigarettes is a complex mixture of chemicals, not pure nicotine, and it is unknown whether the inhalation of such mixtures is safe. Many e-cigarette users, however, reported that the devices helped them to reduce cigarette consumption and even to attain complete abstinence. Following the initial success of a small case series34 and a prospective 6-month pilot study35 with such devices, two landmark RCTs published in 2013 reported positive findings. In the Italian study36 for smokers not intending to quit, e-cigarettes with or without nicotine achieved smoking reduction in 10.3% and complete abstinence in 8.7% of smokers at 52 weeks. In the New Zealand study37 for smokers intending to quit, e-cigarettes with and without nicotine achieved 7.3% and 4.1% continuous abstinence at 6 months respectively, while the NRT patch group achieved 5.8%. The study demonstrated at least noninferiority of nicotine e-cigarettes versus NRT patch. Denicotinised cigarettes, reduced-nicotine cigarettes, and potential reduced-exposure products (PREPs) Denicotinised cigarettes and reduced-nicotine cigarettes (RNCs) are conceptualised under a larger group of tobacco products termed potential reduced-exposure products (PREPs). PREPs are tobaccobased products that claim to reduce the exposure to the toxins found in tobacco. They are not cessation products but are marketed as a way for smokers to continue using tobacco while possibly lessening their toxicant intake.38 Denicotinised cigarettes and RNCs are developed from genetically engineered tobacco, with nicotine reduction in the range of 80-98%.39 Although not marketed for its health benefits, they are now being explored for aiding smoking reduction and cessation. They help smokers to cope with nicotine withdrawal by continuing to provide exposure to the non-nicotine addictive component of tobacco smoke and smoking behaviour. They also dissociate nicotine delivery from the act of cigarette smoking. When used alone, RNCs have been associated with reduced exposure biomarker levels, reduced nicotine dependence and withdrawal scores, and possible substantial abstinence rate.40,41 The use of RNCs with nicotine patch, compared to nicotine patch or denicotinised cigarettes alone, may result in greater satisfaction and craving relief.42-44 RNCs now receive good attention in the US as the FDA is proposing a nationwide gradual reduction in the nicotine content of cigarettes.45 Pharmacogenetics Pharmacogenetics is the study of how the actions of and reactions to drugs vary with the patient's genes. It optimises the selection of treatments for smokers, basing on the individual genotype or phenotype.46,47 For example, clinical trials have shown that NRT patches produce higher abstinence rates in smokers with slower nicotine metabolism by the liver enzyme, CYP2A6. Accordingly, these slow metaboliser smokers might benefit from extended therapy, while subjects with normal rates of nicotine metabolism might be more effectively supported by different therapeutic approaches such as Bupropion. The smoking behaviour and characteristics of each individual have been shown to have strong inheritability. Potential intervention can be directed to specific gene loci including the cholinergic nicotine receptor genes (CHRNB3 and CHRNA5), dopamine pathway genes (DRD2 and DRD4), dopamine transport genes (DAT), serotonin pathway genes such as the tryptophan hydroxylase (TPH), serotonin transporter genes (5HTTLPR), monoamine oxidase genes (MAO-A), dopamine β hydroxylase genes, and genes associated with the metabolism of nicotine such as P540CYP2A6.48 Novel agents Many medications have shown limited efficacy in the treatment of nicotine dependence: Selegiline, Gabapentin, Bromocriptine, Baclofen, Olanzapine, Topiramate, Moclobemide, Venlafaxine, Fluoxetine, Paroxetine, Naloxone, Naltrexone and Mecamylamine. Other agents, such as opiates, anxiolytics, and amphetamines have also been evaluated to be ineffective.46, 49-51 Newer agents that are currently under investigation include: Cytisine52 and Sazetidine-A53, which are partial agonists at the nicotine acetylcholine receptors (nAChRs), UCI-30002, an allosteric nAChR modulator54, and N,N-dodecane-1,12-diylbis-picolinium dibromide, a central nAChR inhibitor.55 Other novel therapeutic approaches not directed at nAChRs include antagonism of the dopamine D3 receptor (for example GSK598809)56, modulation of the glutamatergic system, and inhibition of specific cytochrome P450 (CYP) isoenzymes.
Conclusion In Hong Kong, most smoking cessation centres employ a combination of counselling, NRTs and Varenicline to treat tobacco dependence. Bupropion is not in favour possibly because of its neuropsychiatric adverse event profiles and non-superiority over NRTs. Globally, there is an emerging trend of combining a slow-acting agent (e.g. NRT patch, Bupropion or Varenicline) with an ad libitum agent (e.g. NRT gum, lozenges, nasal spray and inhaler) to manage urges and cravings. E-cigarettes are another fast-growing modality in many countries well received by smokers and non-smokers. Nicotine vaccines, despite their current limited clinical efficacy, are believed to be another promising agent in the years to come. With an ever-improving efficacy of pharmacological agents for smoking cessation, practitioners should again be reminded of the value of other non-pharmacological treatments. There are prevailing guidelines, including those from our Department of Health (accessible at http://www.tco.gov.hk/english/quitting/eresources_for_hcp.html and the US (accessible at http://www.cdc.gov/tobacco/quit_smoking/cessation) for more comprehensive information.
Ling Lo, MBChB (CUHK), FHKCFP, FRACGP, FHKAM (Family Medicine)
Resident Specialist Department of Family Medicine and Primary Health Care, United Christian Hospital, Kowloon East Cluster, Hospital Authority, Hong Kong Tseng-kwong Wong, MBChB (CUHK), FHKCFP, FRACGP, FHKAM (Family Medicine) David VK Chao, MBChB (Liverpool), MFM (Monash), FRCGP, FHKAM (Family Medicine) Correspondence to: Dr Ling Lo, Department of Family Medicine and Primary Health Care, United Christian Hospital, 130 Hip Wo Street, Kwun Tong, Kowloon, Hong Kong SAR, China. References
|
|