|
December 2015, Volume 37, No. 4
|
Update Article
|
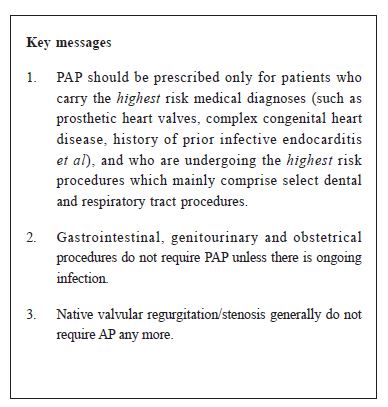
Antibiotic prophylaxis to prevent infective endocarditisArchie Ying-sui Lo 羅鷹瑞 HK Pract 2015;37:143-145 Summary Use of pre-operative antibiotic prophylaxis (PAP) against infective endocarditis (IE) has often been overly enthusiastic. Most guidelines since 2008 have endorsed a major paradigm shift that emphasises restraint in prescribing PAP. PAP should be prescribed only for patients who carry the highest risk medical diagnoses, and who are undergoing the highest risk procedures. Native valvular regurgitation/stenosis generally do not require AP any more. The highest risk conditions include prosthetic heart valves, complex congenital heart disease, prior infective endocarditis and valvulopathy in a transplanted heart, while the highest risk procedures mainly comprise selected dental and respiratory tract procedures. 摘要 過往一向都非常重視在手術前使用抗生素預防感染性心內膜炎。然而,從2008年開始,多數臨床指引都作出指導性改變: 強調需 要節制地 使用術前抗生素。認為預防性抗生素只 適用於最高風險的心臟病患者,和在接受最高風險的外科程式時使用。心瓣膜反流或狹窄,一般不再需要預防性抗生素。屬於最高風險的疾病包括人工瓣膜、複雜發紺性先天性心臟病、曾患感染性心內膜炎和在移植心臟內的心臟瓣膜病;而屬於最高風險的程式主要包括入侵性的牙科(包括洗 牙)和呼吸道手術。除非正值患上感染, 否則腸胃科、泌尿科和產科手術通常無需作術前抗生素預防。 lntroduction In both inpatient and outpatient practices, cardiologists have often received calls from dentists, endoscopists or surgeons enquiring about whether their patients with cardiac conditions, especially those with valvular lesions, require pre-operative antibiotic prophylaxis (PAP). Most cardiologists can still remember the days when PAP was advised for common valvular lesions such as mitral valve prolapse with trivial mitral regurgitation or mild aortic regurgitation. However, the 2007 American Heart Association (AHA) guideline for the prevention of infective endocarditis (IE) was published with a major paradigm shift, supporting the use of PAP only for patients with the highest risk medical conditions undergoing high risk procedures.1 Since 2007, the joint American College of Cardiology/AHA guidelines on the management of valvular heart disease had been updated several times, most recently in 2014.2 The 2009 European Society of Cardiology (ESC) guidelines3 are largely in agreement with the American guidelines. The United Kingdom’s National Institute for Health and Clinical Excellence (NICE) recommended complete cessation of antibiotic prophylaxis for prevention of IE in March 2008.4 After years of monitoring, NICE announced on 1st June 2015 that its original guidance on the use of antibiotics to prevent infective endocarditis will remain unchanged following a review of evidence published since the 2008 guidance.5 The tendency to prescribe PAP may have markedly decreased in America and Europe since 2009. However, it appears that many physicians in Hong Kong still continue to prescribe PAP despite the new guidelines. Clearly the overly aggressive PAP regimens in the past are now deemed inappropriate, because it results in widespread antibiotic resistance, increases medical costs, and causes antibiotic-related side effects. Patients often complain about having to ingest 10 pills all at once (ampicillin). Often, medications for “preventing” gastric discomfort will also be prescribed, thus contributing to polypharmacy. Rationale for the need to exercise restraint in prescribing PAP There has been no human study to-date demonstrating that PAP can prevent endocarditis after invasive procedures. Observational studies have provided, at best, conflicting evidence of treatment benefit.6-9 The 2007 AHA guideline concluded that “bacteraemia resulting from daily activities is much more likely to cause IE than bacteraemia associated with a dental procedure; that only an extremely small number of cases of IE might be prevented by PAP even if prophylaxis is 100% effective; that PAP is not recommended based solely on an increased lifetime risk of acquisition of IE”. PAP can fail as well and IE may still occur despite its administration prior to surgical procedures, even though the majority of the pathogens were susceptible to the PAP agents administered.10 Epidemiologic studies have reported that up to 5% of all cases of IE are preceded by a dental procedure.11 However, despite a close temporal relationship between a dental procedure and the occurrence of IE, it is always difficult to ascertain as to whether IE occurred as a complication of the dental pathology that led to the procedure to begin with, or from routine tooth brushing. Indications for PAP According to the 2007 AHA guideline1, PAP should only be prescribed for patients who carry the highest risk medical diagnoses, and who are undergoing the highest risk procedures (those likely to result in bacteraemia with a microbe that has the potential to cause IE). The AHA guideline considers the following as the highest risk medical conditions:
It should be emphasised that common valvular lesions for which PAP was often prescribed in the past are no longer routine indications for PAP. These include acquired aortic stenosis/regurgitation, mitral stenosis/regurgitation, mitral valve prolapse with or without regurgitation, and bicuspid aortic valve. Obstructive hypertrophic cardiomyopathy is also not an indication any more. The following are considered to be the highest risk procedures:
Conclusion In summary, PAP has often been prescribed indiscriminately and should be reserved only for patients with the highest risk medical conditions undergoing high risk procedures which are likely to result in bacteraemia with a microbe that is potentially pathogenic for IE.
Archie Ying-sui Lo, MD(UChicago), FRCP(Edin), FRCP(Canada), FACC
Honorary Clinical Associate Professor, The Chinese University of Hong Kong Correspondence to: Dr Archie Ying-sui Lo, Room 1103, 11/F, Tower 1, New World Tower, 16 - 18 Queen’s Road, Central, Hong Kong SAR, China
References
|
|