|
June 2017, Volume 39, No. 2
|
Update Article
|
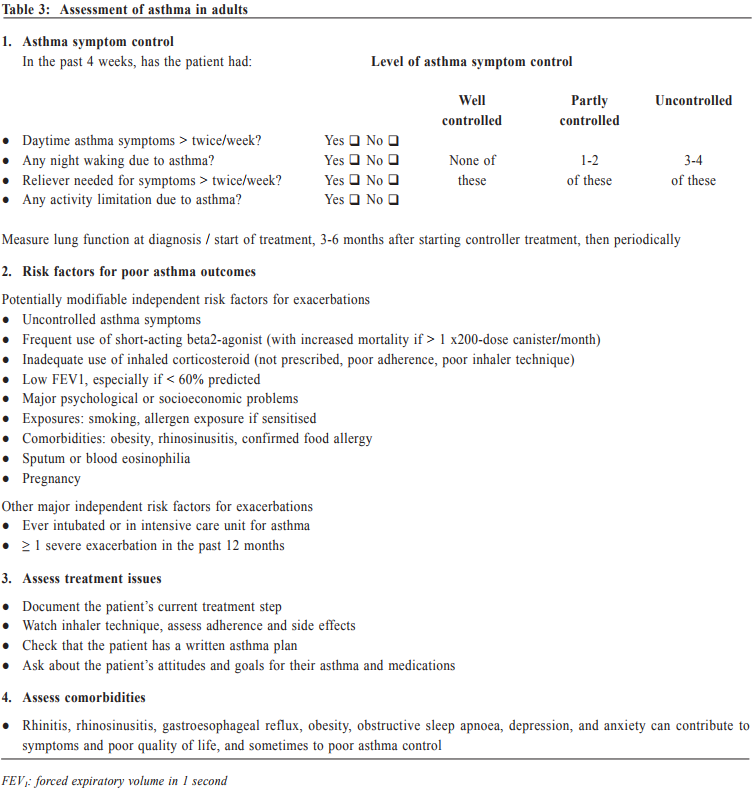
Management of asthma in adults – a general practitioner’s perspectivesVeronica L Chan 陳利 HK Pract 2017;39:43-52Summary Asthma is a heterogeneous airway disease that imposes a major burden for patients, their families, the health care system and the society. In the recent few years, there are rapidly evolving understanding of the disease, broader evidence-based discussions and increasing interest in personalised treatment. The Global Initiative for Asthma (GINA) strategy report has provided guidelines for asthma management. This article aims to summarise the 2016 updated GINA report, with more emphasis on management of asthma in adults from the perspectives of a general practitioner in the primary care setting. Key changes in recommendations include a new diagnosis-centred definition; more emphasis to assess current symptomcontrol as well as future risk; a comprehensive approach for stepwise control-based management that includes the assessment, adjustment and review of treatments that tailored to the individual patient; and more pharmacological treatment options for patients with severe or uncontrolled asthma. 摘要 哮喘是一種異質性氣管疾病,它對病人、家屬、醫療系統和社會都造成沉重負擔。近年來,全球對哮喘的認識迅速增加,實證治療得到更廣泛的發展,亦更關注個人化的治療方法。全球氣喘創議組織(GINA)的策略報告提供了哮喘診治指引。本文總結2016年最新的GINA報告,從基層醫療醫生的角度,總結成年哮喘病人的治療方法。建議的主要改變包括:(一)一個以診斷為主的新定義;(二)更著重評估現有病徵和未來風險;(三)全面的階梯式治療法,包括評估、調整和治療方法回顧以切合病人的個性化需要;(四)為重度或失控的哮喘病人提供更多藥物治療選擇。 Introduction Asthma is a serious global health problem affecting all age groups, with global prevalence ranging from 1- 21% in adults.1 The GINA was established in 1993 by the National Heart, Lung and Blood Institute and the World Health Organisation, and has been revised regularly since 2002, with the goals of disseminating the most up-to-date and evidence-based information to improve asthma care. Based on the latest 2016 GINA report2, this article aims to summarise the updates on management of asthma in adults, with emphasis on the perspectives of a general practitioner in the primary care setting. Definition of asthma Asthma is a heterogeneous disease, usually characterised by chronic airway inflammation. It is defined by the history of respiratory symptoms such as the wheeze, shortness of breath, chest tightness and cough that vary over time and in intensity, together with variable expiratory airflow limitation. Diagnosis of asthma emphasises identification of characteristic symptom patterns, preferably before commencing treatment, with clear documentation in the patient’s medical records. Physical examination in people with asthma often results in normal findings. Otherwise, the most frequent abnormality is expiratory wheezing on auscultation; but this may even be absent during severe asthma exacerbations. Confirmation of diagnosis is achieved by the demonstration of the presence of variable airflow limitation from spirometry with bronchodilator reversibility testing or twicedaily peak expiratory flow (PEF) test. The diagnostic criteria for lung function variation are illustrated in Table 1 and the diagnostic flow chart for asthma on initial presentation is shown in Figure 1. Occasionally, repeat spirometry after a course of anti-inflammatory treatment, exercise-challenge test, or histamine bronchial challenge test may be needed in a specialist setting to minimise under- or over-treatment. It is also very important to differentiate asthma from other airway diseases like upper airway dysfunction, chronic obstructive pulmonary disease (COPD), respiratory infections, tracheomalacia, inhaled foreign body and many other conditions with similar symptoms of dyspnoea and wheezing (Table 2). Assessment of asthma For every patient, assessment of asthma should include the assessment of symptom control, identification of risk factors of adverse asthma outcome, assessment of treatment issues particularly inhaler technique and adherence, as well as assessment of comorbidities that could contribute to symptom burden and poor quality of life (Table 3). Asthma symptoms alone are not sufficient for assessing asthma because symptoms can be controlled by inappropriate use of short or long-acting beta2-agonist alone, which leaves airway inflammation untreated. Some patients, especially elderly, may have few symptoms despite having poor lung function.
Asthma treatment The long-term goals of asthma management are to achieve good symptom control whilst to maintain normal activity levels, and to minimise future risk of exacerbations, fixed airflow limitation and side effects of treatment. GINA emphasises a stepwise control-based asthma management, in which pharmacological and non pharmacological treatment are adjusted step by step, in a continuous cycle that involves patient assessment, adjusting treatment and review (see Table 4). Patients with asthma should be reviewed regularly to monitor their symptom control, risk factors and occurrence of exacerbations. Persistent uncontrolled asthma symptoms are commonly due to poor inhaler technique3, poor medication adherence4, incorrect diagnosis of asthma, comorbidities or complicating conditions such as rhinosinusitis, gastroesophageal reflux, obesity and obstructive sleep apnoea, and ongoing exposure to the effects of smoking, beta-blockers, non-steroidal anti-inflammatory drugs, occupational or domestic allergen exposure. If there are persistent symptoms and exacerbations despite adequately addressing these factors, stepping up in treatment may be recommended. Any stepping up should be regarded as a therapeutic trial, and the response reviewed after 2 to 3 months. If there is no response, treatment should be reduced to the previous level and an alternative treatment option be considered. Indications for referral for specialist care are summarised in Table 5. Once good control has been achieved and maintained for 3 months and lung function has reached a plateau, treatment can often be reduced to find the patient’s minimum effective controller treatment. Generally speaking, reducing the dose of inhaled corticosteroid by 25-50% at 3-months intervals is feasible and safe for most patients.5 Discontinuation of LABA might lead to deterioration6 and complete cessation of ICS is not advised.7 The key to successful step-down is to engage the patient in the process, provide clear instructions, provide written asthma action plan, ensure the patient has sufficient medication to resume their previous dose if the need arises, monitor symptoms and/or PEF, and schedule a follow-up visit.
Personalised treatment There has been increasing interest in the concept of ‘personalised treatment’ of asthma. Nowadays, there are a wide choice of pharmacological agents of inhaled corticosteroid, fixed-dose combination of inhaled corticosteroid and long-acting beta2-agonist, and inhaler devices. Choice of reliever medication has been expanded to include the use of low dose ICS/formoterol as both maintenance and reliever treatment for atrisk patients. This has shown to significantly reduce exacerbations while providing similar levels of asthma control.8 Tiotropium (long-acting muscarinic antagonist) by mist inhaler may be used as add-on therapy for adults or adolescent patients with inadequate control and a history of exacerbations.9 In clinical practice, the choice of medication, device and dose should be based on the assessment of symptom control, risk factors, patient preference, and practical issues (cost, ability to use the device, and adherence). Special investigation may be arranged in a specialist setting to further stratify patients for specific treatment. If patients’ history is suggestive of possible allergen sensitisation, skin prick test or radioallergosorbent test (RAST) can be performed to detect specific IgE to aeroallergens (such as house dust mite, cockroach, fungal molds, cat, dog, etc) or food allergen (such as peanut, egg, milk, etc). Appropriate allergen avoidance and/or allergen immunotherapy10,11 can provide substantial symptom improvement. Patients having moderate or severe allergic asthma that is uncontrolled with at least 2 controller mediations (i.e. Step 4 treatment) can be referred to a specialist centre for further test for biomarkers. Those having high blood IgE level can be considered for add-on omalizumab (anti-immunoglobin E) treatment12, whilst those having high eosinophils in either blood or induced sputum can be considered for add-on mepolizumab (anti-interleukin 5) treatment.13,14 In centres with specific expertise in inducing and analysing sputum, adjusting treatment for severe asthma on the basis of sputum eosinophils may allow significant reduction in corticosteroid dose and/or exacerbation frequency.15
Asthma exacerbations Exacerbations of asthma are episodes characterised by a progressive increase in symptoms (e.g. shortness of breath, cough, wheezing or chest tightness), and progressive decrease in lung function, which requires a change in treatment. All patients should be advised to have regular monitoring of symptom and/or peak expiratory flow, and to be provided with a guided self-management action plan so that they can respond appropriately in the event of worsening asthma. This action plan should have clear instructions regarding how to increase reliever medication, early increase in controller medication, and when to start oral corticosteroid (Table 6). They should be advised to have immediate medical attention if their asthma continues to deteriorate despite following their written action plan, or if their asthma suddenly worsens. When an asthmatic patient with acute exacerbation presents himself in a primary care setting, the physician should conduct a brief focused history and relevant physical examination. The history should include: i) the details of the present exacerbation (onset, precipitating cause if known, symptoms, any exercise limitation and sleep disturbance); ii) the level of asthma control and any risk factors for asthma-related death or poor asthma outcome; iii) treatment issues, particularly inhaler technique and adherence, any recent dose changes and response to current therapy; iv) any comorbidities and associated symptoms. During physical examination, it is important to assess vital signs and exacerbation severity (e.g. level of consciousness, temperature, pulse rate, respiratory rate, blood pressure, ability to complete sentences, use of accessory muscle, and wheeze). Special attention should be made to look for complicating factors (e.g. pneumonia, pneumothorax, anaphylaxis) and signs of alternative diagnosis (e.g. cardiac failure, upper airway dysfunction, inhaled foreign body or pulmonary embolism). Pulse oximetry and peak expiratory flow are also helpful to identify patients with a need for more aggressive therapy. Flow chart for assessment and management of patient with asthma exacerbation is shown in Figure 2. Patient discharge Patients can be discharged if their symptoms improve with less requirement of frequent SABA, their PEF returning to > 60-80% of personal best, satisfactory oxygen saturation and vital signs. Discharge medications should include as-needed reliever medication, oral corticosteroid, regular controller treatment, together with adequate review on inhaler technique and adherence. A follow-up appointment should be arranged in about 2 to 7 days, depending on the clinical and social context. At the review visit, the primary care physician should assess the patient’s level of symptom control, drug compliance, and adjust the treatment plan, either to step up or step down accordingly. What’s new for primary care? Asthma is now emphasised as a heterogeneous disease with different asthma phenotypes. These phenotypes are recognisable clusters of asthma patients with specific demographic, clinical and/or pathophysiological characteristics. ‘Allergic asthma’ usually has childhood onset of symptoms and strong association of past and/or family history of allergy disease such as eczema, allergic rhinitis, and food or drug allergy. ‘Non-allergic asthma’ does not have or have less association with allergy, with neutrophilic or paucigranulocytic cellular profile in their sputum. Other examples include ‘late-onset-asthma’, ‘asthma with fixed airflow limitation’, and ‘asthma with obesity’. However, to date, no strong relationship has been found between specific phenotypes and particular clinical patterns or treatment responses. The new GINA report is now more clinically focused, with multiple practical tools and flow charts to improve its utility for busy frontline clinicians. Primary care physicians are strongly encouraged to refer to the tables and charts in the report to improve the diagnosis accuracy and to minimise under- or over-treatment. Past asthma control assessment have focused too much on current symptom control (e.g. with the Asthma Control Test). This concept was sometimes interpreted as prompting an automatic step-up in controller treatment if symptoms were not well controlled. The new GINA report recommendations include an expanded list of risk factors for poor asthma outcome, as well as treatment issue and comorbidities (Table 3). The key changes for primary care asthma management emphasise that control-based management should include three components: i) comprehensive assessment of symptoms together with risk factors, treatment issue and comorbidities; ii) adjust treatment (both step-up or step-down) in a stepwise approach for both pharmacological and non-pharmacological therapy (e.g. asthma written plan, smoking cessation, physical activity, weight reduction); and iii) review response every 2 to 3 months, to ensure that disease control is optimised while ineffective or poorly-tolerated treatments would be adjusted promptly.
Conclusion The latest GINA guidelines recommend that treatment should not be based only on symptoms but also on risk factors for exacerbations, side effects of therapy and development of fixed airflow limitation. Asthma management aims at a personalised approach to patient care, taking into account the phenotypic characteristics, biomarkers in blood or sputum, personal background and environment, as well as practical issues such as inhaler technique and patient preference. Implementation of these guidelines in primary care practice could facilitate the reduction of the burden of asthma, both for patients and for health care systems. Veronica L Chan, MBChB, FRCP (Edinburgh), FHKAM (Medicine) Correspondence to: Dr Veronica L Chan, Department of Medicine & Geriatrics, United Christian Hospital, 130 Hip Wo Street, Kwun Tong, Kowloon, Hong Kong SAR, China. Email: chanlv@ha.org.hk
References
|
|