
|
September 2019,Volume 41, No.3
|
Original Article
|
Effectiveness of Hepatitis B immunisation in newborn infants from mothers with Chronic Hepatitis B infection - A Macau experienceIn Wong 王燕,Sio-on U 余兆安,Im-kuan Chan 陳艷群,Chau-sha Kwok 郭秋莎 HK Pract 2019;41:69-76 SummaryObjective: Keywords: Hepatitis B virus infection, Hepatitis B vaccination, Hepatitis B surface antibody, Hepatitis B prevention 摘要目的:澳門曾是乙型肝炎(乙肝)高流行地區,自1989年起,澳門衛生局開展乙肝疫苗的接種計劃,並給予母親為乙肝帶病毒者的高危嬰兒注射乙肝免疫球蛋白。2007年更推出乙肝高危嬰兒隨訪指引。該指引建議母親為乙肝帶病毒者的高危嬰兒在9-15月齡時檢測乙肝表面抗原和表面抗體,如表面抗體為陰性者,需給予另外三劑乙肝疫苗的接種。本研究旨在分析首三劑乙肝疫苗的成效及完成第二輪乙肝疫苗接種後的表面抗體陽性率。
關鍵字:乙型肝炎病毒感染,乙型肝炎疫苗,乙型肝炎表面抗體,乙型肝炎預防 IntroductionHepatitis B virus (HBV) infection is an important global health problem. It is a major cause of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (HCC).1-3 It is prevalent in Asia, Africa, southern Europe, and Latin America, where the Hepatitis B surface antigen (HBsAg) seropositive rates ranged from 8% to 20% before the Hepatitis B vaccine became available in 1982.4 The worldwide updates of the effective vaccine against Hepatitis B have dramatically reduced the carrier rate of HBV and significantly decreased the incidence of childhood HCC.5-8 In highly prevalent areas where the HBsAg seropositive rate is above 8%, primary HBV infections occur mainly during infancy and early childhood. In fact, the rate of chronicity is approximately 90% in infants infected at birth or during the first year of life, 30 - 50% in children aged 1 - 6 years, and 5% - 10% in children above 6 years of age and in adults.9 Children who were not infected at birth remained at significant risk because of long-term interpersonal contact with their infected mothers. In one study, 38% of infants who were born to HBsAg positive mothers and who were not infected perinatally became infected by 4 years of age.12,13 Hepatitis B vaccination is the most effective measure to prevent HBV infection and its consequences. Since they were first issued in 1982, recommendations for Hepatitis B vaccination have evolved into a comprehensive strategy to eliminate HBV transmission in many countries. However, not all infants respond to Hepatitis B immunisation.14-16 Macau used to have a high prevalence of HBV infection. Therefore, universal HBV vaccination with recombinant Hepatitis B vaccine in a three-dose regimen (10 mcg dose at 0, 1, and 6 months) was introduced in 1989, and infants from HBsAg carrier mothers were also given Hepatitis B immunoglobulin (HBIG) immediately after birth. Although the coverage rate of the third dose of Hepatitis B vaccine in Macau was higher than the global coverage rate (95.2% versus 84%)17,18, there was still several new cases of Chronic Hepatitis B infection in infants annually. To improve the effectiveness of Hepatitis B vaccination, the Health Bureau of Macau launched a Follow-up Guideline in 2007 to test the HBsAg and anti-HBs titer level of the high-risk babies at 9 - 15 months of age (Appendix 1). A booster vaccination was given to infants without adequate protective anti-HBs titer levels. According to the recommendation by the Health Bureau in 2007, the non-responders should be reimmunised with recombinant Hepatitis B vaccine immediately with a three-dose regimen (10 mcg dose at 0, 1, and 6 months), and the HBsAg and anti-HBs statuses should be rechecked 3-9 months later if the infants were born in 2007. To effectively implement the 2007 Follow-up Guideline, the Macau Health Bureau has set up a check box about the status of the mother’s Hepatitis B infection in the child health electronic medical record system. The clinician must input the information before saving the child health medical record, otherwise the record cannot be saved. The Macau Health Bureau's electronic medical record system was launched in 2001. After years of continuous updating, the current vaccine registration system has been perfected. Even infants born in private hospitals can record vaccinations through a unified platform. This study was conducted to evaluate the efficacy of HBV vaccination with HBIG at birth and the seroconversion rate after re-immunisation in the infants without adequate protective anti-HBs titer levels. 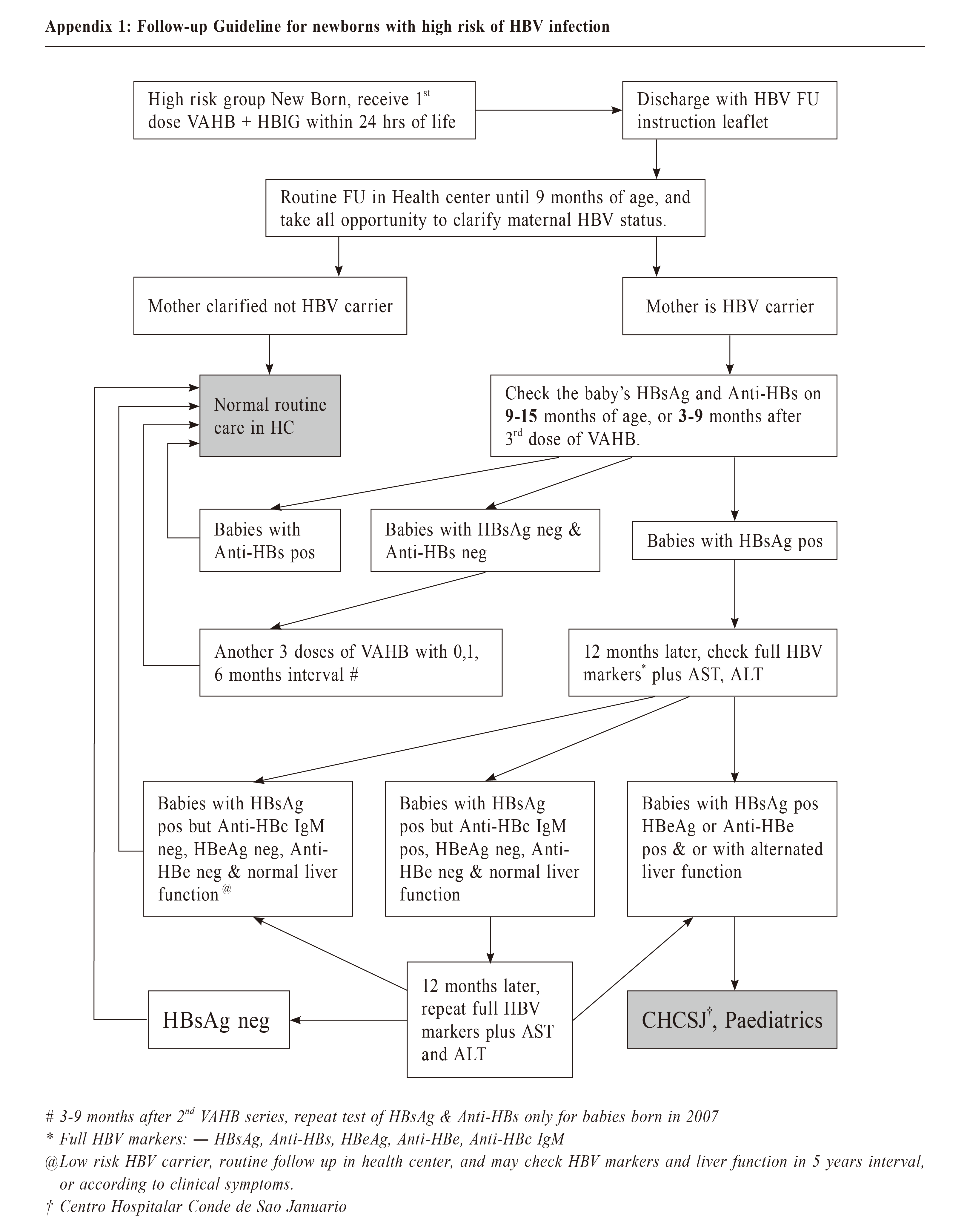
MethodsA retrospective study was conducted by reviewing the computerised medical records of Macau Health Bureau. The inclusion criteria included 1) infants born between 1st January 2007 and 31st December 2017 (irrespective of birth country) 2) infants who received neonatal care service in the primary care setting of the Macau Health Bureau and 3) whose mother were HBsAg positive. All infants that fulfilled the above criteria were included. Based on the 2007 Follow-up Guideline, the infants that fulfilled the above criteria and who had a positive HBsAg and anti-HBs test results at 9-15 months of age were defined as attending follow-up. An anti-HBS titer ≥ 10 mIU/mL was defined as a satisfactory antibody response. The follow-up rate and coverage rate of HBIG and the third dose of the Hepatitis B vaccine were compared between the Macau-born and non-Macau-born infants. Statistical analysisThe categorical variables were expressed as proportions, and these categorical variables in different groups were compared using chi-square tests. P<0.05 was considered to be statistically significant. The percentages were calculated on the basis of the total responses. All statistical analyses were carried out using SPSS version 24 (IBM Corp., Armonk, New York). Ethical issuesThe patients' identities were protected, and no individual patients could be identified from the data obtained. Ethical approval for the study was obtained from the Medical Ethical Committee of Centro Hospitalar Conde de Sao Januario (Reference No: 0968/SCSD/ N/2018). The patients and doctors involved were not at risk of harm because this study was a retrospective study of infants whose mother were HBsAg-positive. ResultsFrom 2007 to 2017, there were 66,960 babies born in Macau. The coverage rate of the third dose of the anti-Hepatitis B vaccine has remained above 90% since 2007, and was over 95% in 2017.17 Over 85% of neonates have registered for the child health service in the government’s primary care setting. Of the infants who received child health services from the outpatient clinics of the Macau Health Bureau, 4705 infants were born to HBsAg-positive mothers. Of these infants, 4475 (95.1%) were born in Macau, and 230 (4.9%) were born in other countries. Of the 230 non-Macau-born infants, 166 (72.2%) were born in mainland China, 33 (14.3%) were born in Hong Kong, 10 (4.3%) were born in the United States, and 23 (9.1%) were born in other countries. Of the 4,705 infants born from HBsAg-positive mothers, 4,515 (96.0%) had computerised records of receiving HBIG within 24 hours of birth, and 4,585 (97.4%) had completed the third dose of anti-Hepatitis B vaccine. Infants born in Macau had a higher coverage rate of HBIG (98.3% versus 50%, p<0.0001) than infants born in other countries, but there were no significant differences in the coverage rate of the third dose of the anti-Hepatitis B vaccine (97.5% versus 97%, p=0.0627) (Table 1). After three doses of anti-Hepatitis B vaccine, 2,428 (51.6%) infants had their HBsAg and anti-HBs titers levels checked at 9 - 15 months of age (Table 1). Compared with non-Macau-born infants, the infants born in Macau had a higher follow-up rate (52.3% versus 37.8%, p<0.0001). And 2,238 (92.2%) out of the 2,428 infants developed a satisfactory antibody response, but unfortunately 47 (1.9%) infants were found to be HBsAg positive, and 143 (5.9%) were non-responders (Figure 1). Of the 143 non-responders, 97 (67.8%) had received the full three-dose booster course, and 14 (7.2%) and 5 (2.6%) had received just two and one booster doses, respectively. In the end, only 50 (35%) infants were re-evaluated for seromarkers, and 47 (94%) of them were noted to have seroconverted with anti-HBs titers >10 mIU/mL after the three-dose booster (Figure 1). The total protective rate finally increased to 94.1%. DiscussionPerinatal immunisation against HBV infection is practiced in many countries. Although the nonresponse rate is low, the non-responders remain susceptible to infection, especially if the mothers has chronic HBV infection. This result is similar to that in other studies of Chinese adults.19,20 In this study, the initial nonresponse rate to primary immunisation with recombinant Hepatitis B vaccine was 5.9%. This rate is similar to that in other studies of infants.21 The current evidence suggests that this lack of response could be caused by a permanent inherent defect (e.g. inability of peptide to bind to certain HLA antigens) or that of a temporary incapacity (e.g. in T helper cell numbers or factors) only present during the perinatal period. This phenomenon could be associated with the relative immaturity of the immunological system at the early stage of extrauterine life. This immaturity disappears with growth and could account for the satisfactory response to the Hepatitis B vaccine after four years. After re-immunisation, the seroconversion rate was high at 94%. This resulted in an increase in the total protective rate from 92.2% to 94.1%. The efficacy is likely to be underestimated because of poor compliance with booster immunisation. If all the non-responders are re-immunised, the estimated protective rate could increase to near 98%. Almost all the mothers were checked for the HBV seromarkers during prenatal care, but only 51.6% of the high-risk infants adhered to the 2007 Follow-up Guideline, even though both services were free to all Macau residents. The unsatisfactory compliance with the 2007 Follow-up Guideline may be due to the following reasons: (a) infants only live in Macau for a short period of time, partly because their mothers were not Macau residents, but were only in Macau for short-term work, and partly because of the low cost of hospitalisation in Macau government hospitals. Women in neighboring areas give birth in Macau as tourists, (b) the infants at high risk were missed in a busy clinical practice, (c) the 2007 Follow-up Guideline was not widely promoted, and some doctors were not aware of it or considered it a research project only. An automated reminder of the high risk for these infants in the computerised medical record system of the Health Bureau could be helpful. This study found that the rate of non-Macau-born infants participating in follow-up was significantly lower than that of infants born in Macau, mainly because most of the infants were new immigrants. The age at which they came to Macau exceeded the age recommended by the guidelines. Therefore, no follow-up was performed. 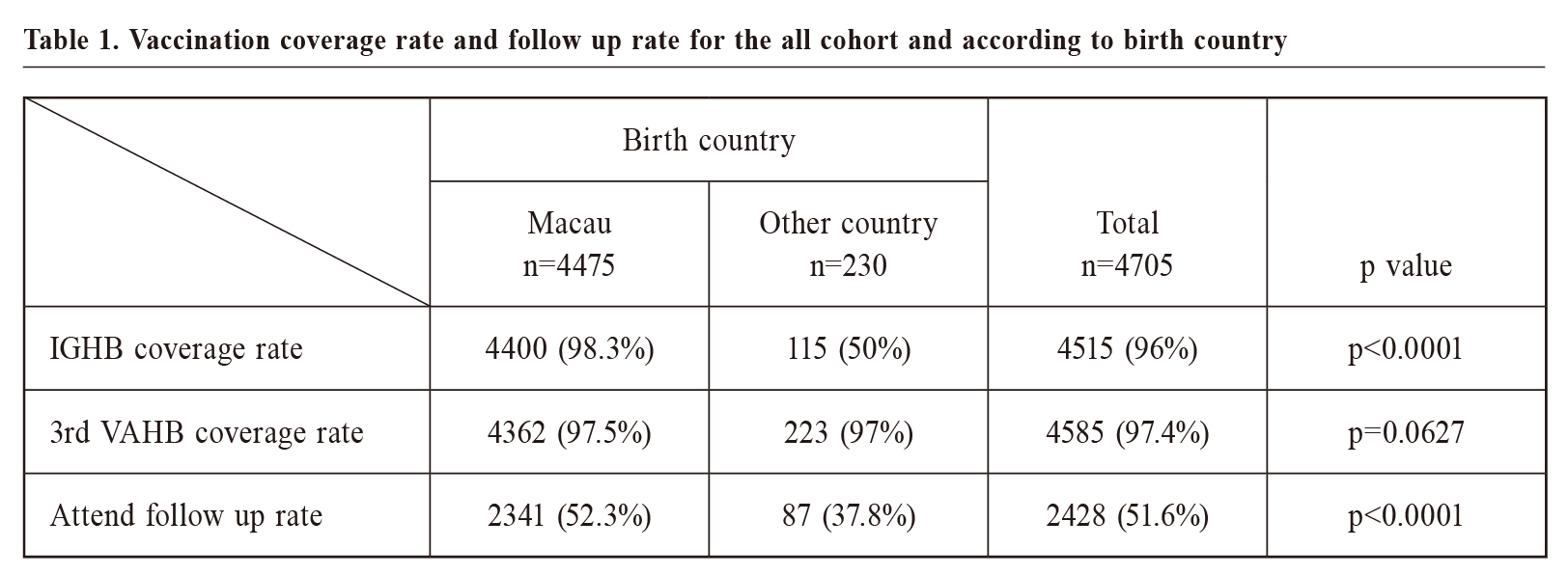 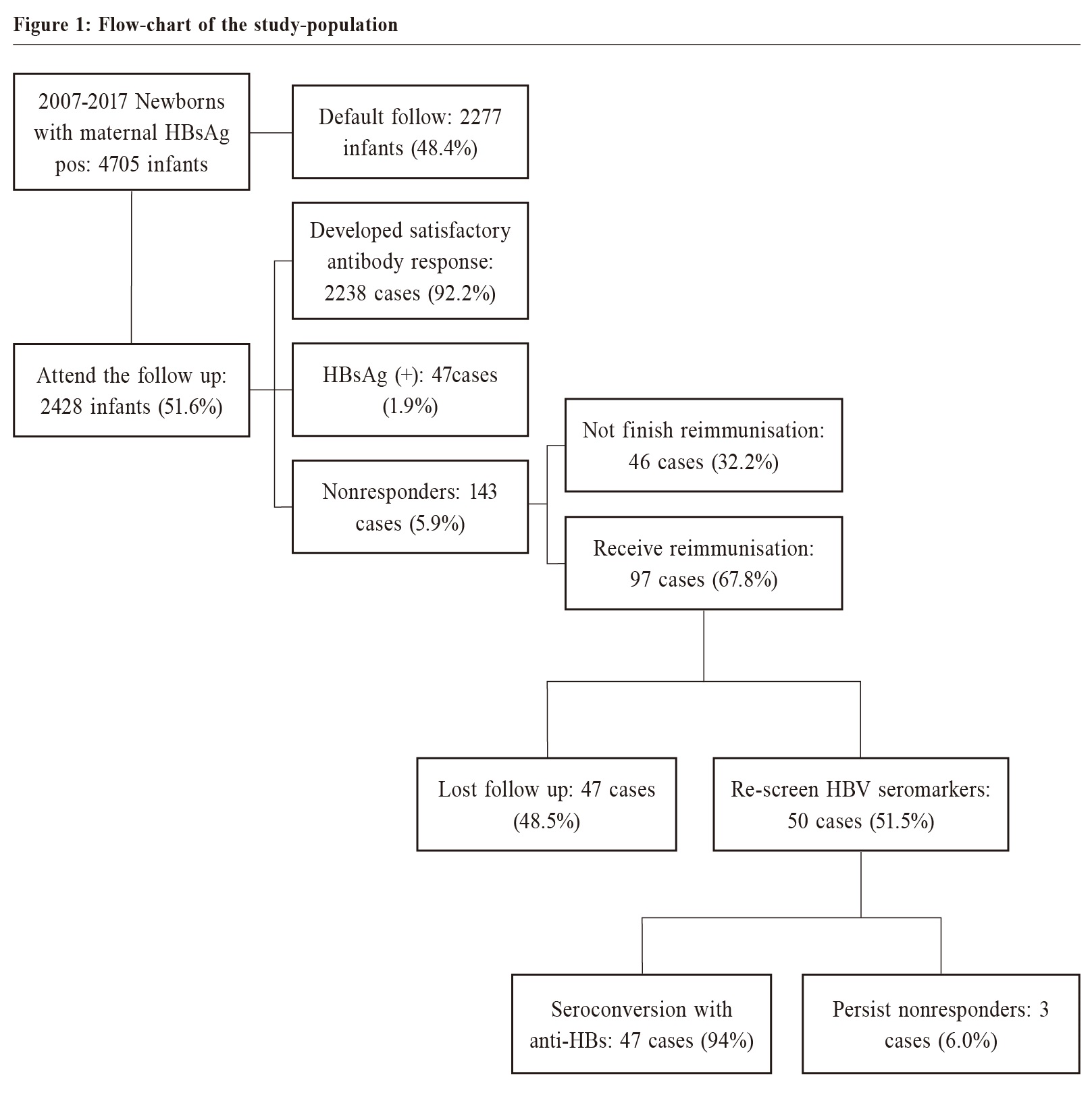 Limitations of the StudyThis study reviewed the information on all registered child health care in the electronic medical record system of the Macau Health Bureau over the past decade. Although the follow-up rate was low, it covered most of the data of Macau residents. One of the limitations of this study was that most of the personal information from the cases that did not participate in the follow-up was incomplete, so the specific reasons for the low follow-up rate and their proportion could not be analysed. Another limitation was that the low follow-up rate may underestimate the effectiveness of the 2007 Follow-up Guideline.  ConclusionDue to the serious complications of Chronic Hepatitis B infection contracted during the perinatal period, checking the anti-HBs titer level after the primary immunisation is to be warranted in high-prevalence areas. Re-immunisation as laid out by the 2007 Follow-up Guideline increases the protective rate and identifies the infected baby for future management. Relevant reminders in the computerised medical record systems could improve the execution of these guidelines.
In Wong,Fellow of Macao Academy of Medicine (MAM) (Family Medicine)
Correspondence to:Dr In Wong, Sao Lourence Health Center, Health Bureau of Macau SAR, China
References:
|
|