Recognising familial hyperlipidaemia in
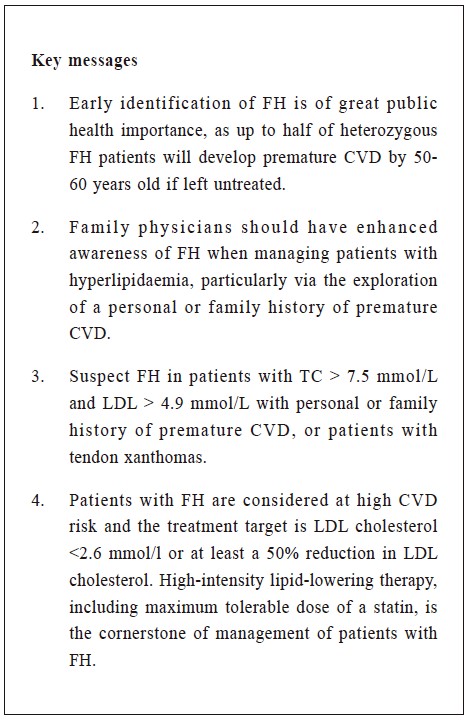
adult patients in primary care
Dorcas Yan 甄多嘉, Kwai-sheung Wong 黃桂嫦, Catherine XR Chen 陳曉瑞
HK Pract 2023;45:97-101
Summary
Despite being one of the most common genetic
disorders, familial hyperlipidaemia (FH) remains
underdiagnosed both locally and internationally. Due
to its drastic consequences such as fatal premature
cardiovascular events, timely recognition and
management of FH may significantly improve the
clinical outcomes of patients with FH.
As the first contact and gate keeper of the
healthcare system, family physicians need to have
enhanced awareness of FH when managing patients
with hyperlipidaemia, and offer intensive interventions
to reduce the patients’ cardiovascular risk.
摘要
家族性高膽固醇血症是臨床上最常見的遺傳病之一,
但在本地和國際上的關注不多,以致很多個案未被明確診
斷出來。家族性高膽固醇血症可導致致命性心血管疾病等
嚴重後果,而及時診斷和治療本病可明顯改善病人的臨床
成效。作為醫療系統的守門人,家庭醫生需要提升對此病
的關注,及時診斷並提供積極有效的治療,以減低病人患
心血管疾病的風險。
The Case
We report here a case of familial hyperlipidaemia.
Our patient, Ms. Chung (not her real name), was
75-year-old in 2021; and she had been attending at our
government primary care clinics for many years. She
had had hypertension, primary hypothyroidism and
hyperlipidaemia since 2018. Her medications included
Rosuvastatin 20 mg daily, Amlodipine 7.5 mg daily,
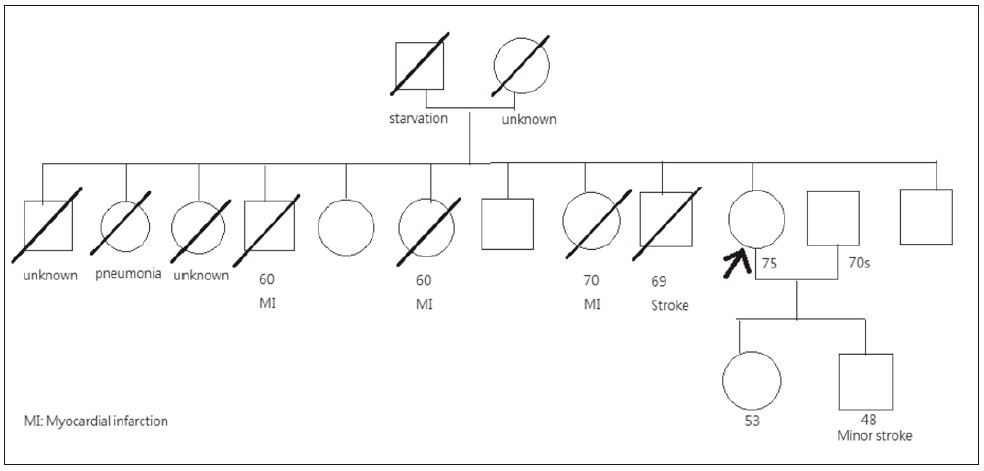
and Thyroxine 25 mcg daily. She had a strong family
history of cardiovascular diseases (CVD), with 4 out
of her 12 siblings being affected either by myocardial
infarction or stroke. Also, her son suffered from a minor
stroke in his 40s (Figure 1).
Ms. Chung is still attending our follow-up clinic
at the moment. However, she was seen in our General
Outpatient Clinic (GOPC) of the Hospital Authority in
August, 2021 for skin nodules for 3 months. The skin
nodules first appeared on her elbows on both sides,
which then extended to her forearms, knees and down to
her feet dorsum. The nodules were not associated with
pain or itchiness. There was no skin rash, joint pain, or
fever, neither history of gout nor rheumatoid arthritis.
There was no recent special contact history and she
had not taken any herbal medicine or drugs from over
the counter. Her blood pressure had been satisfactorily
controlled all along with good drug compliance. Up
till now, she has no chest pain or shortness of breath,
and has maintained a healthy diet and lifestyle. She has
never smoked nor consumed alcohol.
In 2021, her general condition had been good.
Physical examination was satisfactory. Her clinic blood
pressure (BP) was 128 / 76 mmHg, pulse 76 beats per
minute, and she was not obese clinically.
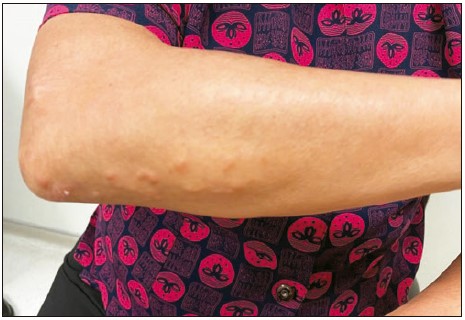
There were crops of erythematous papules over the
extensor surfaces of both her forearms and knuckles,
sparing the palmar crease (Figure 2). There was no
arcus cornealis or xanthelasmas. At the time of her
visit, clinically she was euthyroid.
Figure 1:
Family tree of Ms. Chung
Figure 2:
Picture of the skin lesion (with the kind
permission of Ms. Chung). There were crops of
erythematous papules over the extensor surfaces
of both forearms, which were consistent with
the diagnosis of tendon xanthomas.
Lipid profile done in June, 2018 when she was first
diagnosed with hyperlipidaemia was: total cholesterol
(TC) 8.5 mmol/L, high-density lipoprotein (HDL) 1.5
mmol/L, low-density lipoprotein (LDL) 6.2 mmol/L,
and triglycerides (TG) was 1.7 mmol/L. Her liver, renal,
thyroid function tests and fasting sugar level were all
normal. She was advised to have lifestyle modifications
and Rosuvastatin 20 mg was prescribed for the lipid
control. Blood tests in July, 2021 showed that TC was
down to 6.6 mmol/L, HDL was 1.6 mmol/L, LDL was
4.5 mmol/L, and TG was 1.1 mmol/L. Annual ECG
examination showed normal sinus rhythm without
ischemic changes.
In light of the strong family history of CVD, the
typical physical examination findings and the very
high LDL level, the most likely diagnosis of the skin
nodules for this lady was cutaneous xanthomas due
to her underlying disease of familial hyperlipidaemia
(FH). Hence, Rosuvastatin dose was stepped up to 40
mg daily, and the patient was reinforced on lifestyle
modifications.
Year 2021
Her lipid profile in November, 2021 showed that
TC was 5.0 mmol/L, HDL was 1.6 mmol/L, LDL was
3.0 mmol/L, and TG was 1.1 mmol/L. As the LDL level
remained suboptimal, self-financed Ezetimibe (FDAapproved
in 2002) 10 mg daily was added on top of
Rosuvastatin to intensify the LDL control. Her latest
blood tests in March, 2022 showed a satisfactory lipid
control with TC 3.6 mmol/L, HDL 1.7 mmol/L, LDL
1.4 mmol/L, and TG 0.9 mmol/L. Ms. Chung was
arranged to have continued follow up (FU) with regular
monitoring of the lipid profile.
Discussion
Heterozygous familial hyperlipidaemia (HeFH)
is one of the most common genetic disorders in the
general population across the world, with a prevalence
ranging from 1: 200 to 1:311.1,2 The prevalence of
HeFH in Hong Kong remains unknown, but large population studies from mainland China using the
Chinese modified Dutch Lipid Clinic Network (DLCN)
definition revealed that the crude prevalence of HeFH
was 0.28% to 0.35%3,4, which is slightly lower than
that of the western population. HeFH is an autosomal
dominant condition caused by genetic mutations
related to the LDL receptor pathway, with high levels
of LDL leading to the premature development of
atherosclerotic cardiovascular disease. If left untreated,
men and women with HeFH typically develop coronary
heart disease before the ages of 55 and 60 years,
respectively; 50% of men and 15% of women die
before these ages.5,6 Despite its prevalence and potential
severe consequences, FH remains underdiagnosed and
undertreated globally.6,7 Therefore, concerted efforts
among all health care workers, particularly in primary
care, should be made to enhance the awareness of FH.
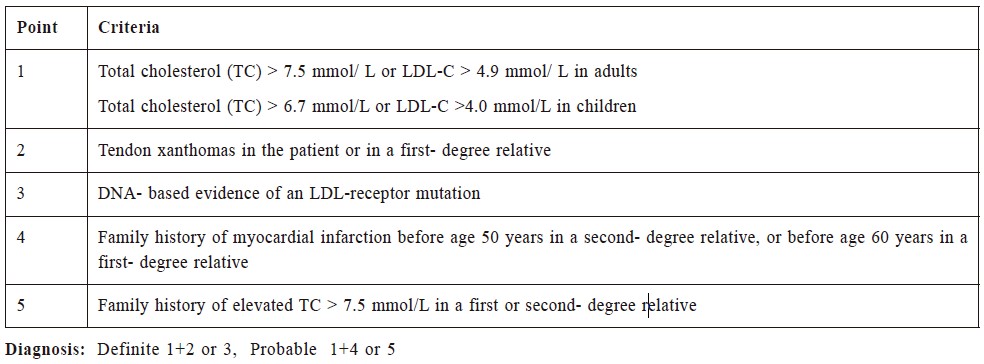
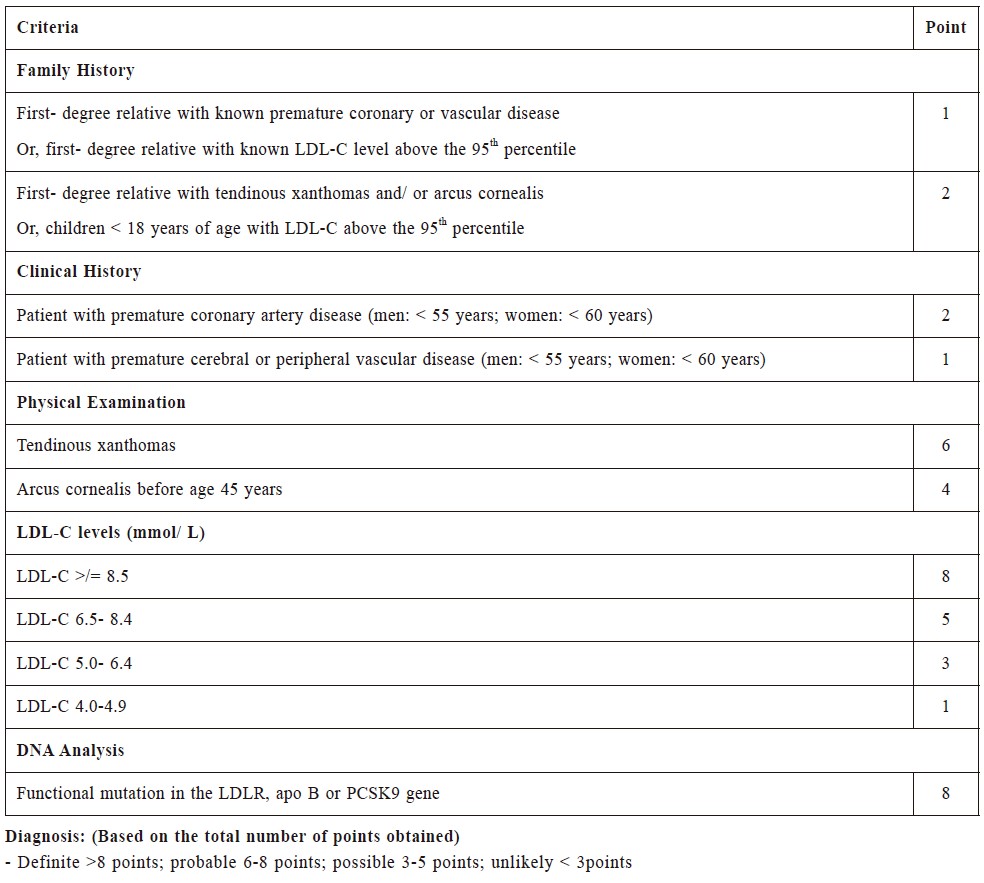
A number of diagnostic criteria for FH have been
reported in the literature, such as the Simon Broome
Register Diagnostic Criteria and the DLCN Diagnostic
Criteria (Table 1 & 2).8,9 There is no consensus on
which set of criteria is superior than the other. The
diagnosis of FH mainly takes into account the patient's
personal history, family history, clinical signs such as
tendon xanthomas and arcus cornealis, as well as the
much elevated LDL levels.10
Tendon xanthomas are white or yellow cholesterol
deposits over the extensor tendons, typically the
Achilles, subpatellar and hand extensor tendons.
They are considered pathognomonic and specific
for the diagnosis of FH.10,11 Secondary causes of
hyperlipidaemia including hypothyroidism, nephrotic
syndrome, obstructive liver diseases, steroid use, and
excessive alcohol intake should be ruled out before the
diagnosis of FH is established.12
In general, physicians should consider the
possibility of FH in patients with premature coronary
events and whose TC is over 7.5 mmol/L, or LDL is
over 4.9 mmol/L.2,9,12
Local guideline recommend a lower LDL
threshold- the possibility of FH should be considered
in patients with family or personal history of premature
coronary events and LDL > 4.5 mmol/L.11 Our patient
Ms. Chung fulfilled the Simon Broome Register
Diagnostic Criteria of definite FH based on her strong
family history of CVD, in particular two of her siblings
dying of myocardial infarction at 60 years old, the presence of tendon xanthomas over her forearms, as
well as her sky-high TC and LDL level (8.5 mmol/
L and 6.2 mmol/L respectively) before treatment.
She also fulfilled the DLCN Diagnostic Criteria of
definite FH with a total score of 10. The otherwise
normal laboratory findings excluded the possibility of a
secondary cause accountable for her hyperlipidaemia.
Diagnosis of FH is often made based on the clinical
information and the laboratory findings. Although
genetic testing is usually not needed, a positive
genetic test with FH gene mutation is associated with
a significantly higher cardiac risk.10 Locally, genetic
testing is available at Clinical Genetic Service of
the Department of Health11 or in advanced private
diagnostic centers. CVD risk assessment tools such as
those based on the Framingham algorithm should not be
used for people with FH. This is particularly important
to note for healthcare professionals in the primary care
setting as patients with FH are already considered as
having a high risk of developing CVD.
Multiple factors modify the risk of HeFH, such
as male sex, smoking, presence of diabetes mellitus,
hypertension, subclinical coronary atherosclerosis, lower
HDL and higher lipoprotein(a) levels.13 Our patient Ms.
Chung has not been diagnosed to have HeFH until she
is 75 years old and fortunately she has not suffered
from a CVD attack until this moment. Female gender,
well controlled BP, her healthy lifestyle and lack of
other CVD risk factors such as smoking or diabetes
mellitus might have helped to reduce her overall CVD
risk. Indeed, HeFH is easily missed or underdiagnosed
in primary care. All family physicians are advised
to enlist HeFH as one of the differential diagnoses
whenever a dyslipidaemia patient is encountered.
Timely and effective lipid control improves the life
expectancy of patients with FH. The management of
FH consists of counselling, family screening, lifestyle
advice, treatment of CVD risk factors and intensive
lipid-lowering therapy starting early in life. Physicians
could counsel patients regarding the implications and
mode of inheritance of FH. Family screening should
be offered as half of the patient’s first-degree relatives
could be affected.5 Screening can be done by measuring
the LDL levels, carrying out genetic analyses, or both.7,8
Apart from counselling and family screening, physicians
should promote positive lifestyle changes, advocating
for healthy diet, regular exercises, weight reduction, and
Table 1:
Simon Broome Diagnostic Criteria for familial hypercholesterolemia.
Table 2:
Dutch Lipid Clinic Network Diagnostic Criteria for familial hypercholesterolemia
smoking cessation if the patient smokes.9 High-intensity
lipid-lowering therapy, such as maximum tolerable
dose of statin, is the cornerstone of FH management.
Ezetimibe is considered as second line treatment if
LDL fails to be adequately controlled by statin alone9,11,
and bile acid sequestrants or niacin are considered
as third line choices.7 If these agents are exhausted,
patients could be referred to specialists for considering
proprotein convertase subtilisin/kexin 9 (PCSK9)
inhibitors treatment.11,12
With regard to the target of LDL control, most
guidelines recommend a LDL target of below 2.6 mmol/
L, or at least a 50 % reduction in LDL cholesterol for
the primary prevention of CVD.7 A LDL target of below
1.8 mmol/L is used for secondary prevention in patients with established CVD.11 Australian and European
Society of Cardiology guidelines suggest a stricter
target of LDL < 1.4 mmol/L for patients with clinical
evidence of atherosclerotic CVD.7,12
For our patient Ms. Chung, her LDL level of 4.5
mmol/L in July, 2021 was apparently not adequately
controlled and therefore Rosuvastatin dose was stepped
up. After the dose augmentation, her LDL level had
improved, though remained suboptimal. To further
reduce her CVD risk, Ezetimibe was added on top of
the statin treatment, which successfully brought her
LDL level down to target. Ms Chung’s clinical condition
will be regularly reviewed and her lipid profile will be
closely monitored in future FUs.
Referencess
-
Hu P, Dharmayat KI, Stevens CAT, et al. Prevalence of familial
hypercholesterolemia among the general population and patients with
atherosclerotic cardiovascular disease: A systematic review and metaanalysis.
Circulation. 2020;141(22):1742–1759.
-
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the
management of dyslipidaemias: lipid modification to reduce cardiovascular
risk. Eur Heart J. 2020;41(1):111–188.
-
Wang Y, Li Y, Liu X, et al. The prevalence and related factors of familial
hypercholesterolemia in rural population of China using Chinese modified
Dutch Lipid Clinic Network definition. BMC Public Health. 2019;19(1):837.
-
Shi Z, Yuan B, Zhao D, et al. Familial hypercholesterolemia in China:
prevalence and evidence of underdetection and undertreatment in a
community population. Int J Cardiol. 2014;174(3):834–836.
-
Brett T, Arnold-Reed D. Familial hypercholesterolaemia: A guide for general
practice. Aust J Gen Pract. 2019;48(9):650–652.
-
Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial
hypercholesterolaemia is underdiagnosed and undertreated in the general
population: guidance for clinicians to prevent coronary heart disease:
consensus statement of the European Atherosclerosis Society. Eur Heart J.
2013;34(45):3478–3490a.
-
Lui DTW, Lee ACH, Tan KCB. Management of familial hypercholesterolemia:
Current status and future perspectives. J Endocr Soc. 2021;5(1):bvaa122.
-
Tan K, Cheung CL, Yeung CY, et al. Genetic screening for familial
hypercholesterolaemia in Hong Kong. Hong Kong Med J. 2018;24 Suppl
3(3):7–10.
-
Recommendations. Familial hypercholesterolaemia: identification and
management. Guidance. NICE. [cited 2021 Dec 26]; Available from:
http://www.nice.org.uk/guidance/cg71/chapter/Recommendations
-
McGowan MP, Hosseini Dehkordi SH, Moriarty PM, et al. Diagnosis and
treatment of heterozygous familial hypercholesterolemia. J Am Heart Assoc.
2019;8(24):e013225.
-
Tomlinson B, Chan JC, Chan WB, et al. Guidance on the management of
familial hypercholesterolaemia in Hong Kong: an expert panel consensus
viewpoint. Hong Kong Med J. 2018;24(4):408–415.
-
Brett T, Radford J, Heal C, et al. Implications of new clinical practice
guidance on familial hypercholesterolaemia for Australian general
practitioners. Aust J Gen Pract. 2021;50(9):616–621.
-
Rocha VZ, Santos RD. Past, present, and future of familial hypercholesterolemia
management. Methodist Debakey Cardiovasc J. 2021;17(4):28–35.
Dorcas Yan,
MBBS (HK)
Resident,
Dept. of FM and GOPCs, Kowloon Central Cluster, Hospital Authority
Kwai-sheung Wong,
MBBS (HK), FHKAM (Family Medicine)
Associate Consultant,
Dept. of FM and GOPCs, Kowloon Central Cluster, Hospital Authority
Catherine XR Chen,
LMCHK, PhD (Med, HKU), MRCP (UK), FHKAM (Family Medicine)
Consultant,
Dept. of FM and GOPCs, Kowloon Central Cluster, Hospital Authority
Correspondence to: Dr. Dorcas Yan, Room 807, Block S, Queen Elizabeth Hospital,
30 Gascoigne Road, Kowloon, Hong Kong SAR.
E-mail: yd902@ha.org.hk
|