Utilisation rate of non-vitamin K antagonist oral
anticoagulant, and associated factors of refusal
of non-vitamin K antagonist oral anticoagulant
usage in atrial fibrillation patients - A study
in two Hong Kong general out-patient clinics
Liujing Chen 陳柳靜, Chik-pui Lee 李植沛, Lit-ping Chan 陳列萍, Eric MT Hui 許明通, Maria KW Leung 梁堃華
HK Pract 2023;45:89-96
Summary
Objective:
To evaluate the utilisation rate of non-vitamin
K antagonist oral anticoagulant (NOAC) and associated
factors of NOAC refusal in atrial fibrillation (AF) patients.
Design:
A cross-sectional study.
Subjects:
All the AF patients, who were regularly
followed up in two public general out-patient clinics
(GOPC) from November 2019 to March 2020, aged
older than 18 years, and eligible for NOACs.
Main outcome Measures:
Utilisation rate of NOAC in
AF patients. The associated factors of NOAC refusal in
AF patients.
Results:
A total of 324 patients were included during
the study period. Utilisation rate of NOAC in AF patients
was 54%. Multivariate analysis revealed that older age,
higher financial strain score, lack of sponsor for NOAC
and lower CHA2DS2-VASc score were the factors
that were significantly associated with NOAC refusal.
Conclusion:
In our study, the utilisation rate of NOAC in
AF patients in the two local GOPCs studied was 54%,
which implied there was still room for improvement.
The associated factors of NOAC refusal highlighted
the importance of financial support to promote the
usage of the NOAC. Further research and strategy
to improve guideline attainment should focus on the
subgroup patients who were older and who had a lower
CHA2DS2-VASc score.
Keywords:
Atrial fibrillation, anticoagulation, non-vitamin
K antagonist oral anticoagulant, Hong Kong,
primary care
摘要
目的 :
評估非維他命K拮抗劑類口服抗凝血劑(NOAC)在心
房纖顫(AF)患者中的使用率,並且分析患者拒絕此藥的相關
因素 。
設計 :
橫斷面研究。
對象 :
2019年11月至2020年3月於兩間普通科門診常規複診
的AF成年患者,並且符合NOAC使用指徵。
主要測量內容 :
NOAC在AF患者中的使用率,以及患者拒
絕此藥的相關因素。
結果 :
共324位患者加入研究。NOAC在AF患者中的使用
率為54%。多因素分析結果顯示年老、高財務壓力評分、
NOAC缺乏資助、以及CHA2DS2-VASc低分是拒絕NOAC的
相關因素。
結論 :
本研究顯示,NOAC於兩間本地普通科門診AF患者
中的使用率為54%,仍然有待提高。相關因素分析顯示:
資助NOAC對於提高其使用率相當重要;對於年老以及
CHA2DS2-VASc分數低的患者,仍然需要進一步的分層分
析,尋找提高他們使用NOAC的途徑。
關鍵詞 :
心房纖顫,抗凝血劑,非維他命K拮抗劑類口服
抗凝血劑,香港,基層醫療
Introduction
Background and objectives
Atrial fibrillation (AF) is the most common cardiac
arrhythmia1, with a lifetime prevalence of one fourth of
patients >40 years old.2 In a Hong Kong territory-wide
community-based AF screening programme, the prevalence
of AF detected by smartphone-based wireless single-lead
ECG or self-reported by participants was 8.5%.3
Individuals with AF have an increased risk of
stroke, and account for up to 1 of 3 stroke cases among
the elderly4,5, potentially leading to permanent disability
and death.6 Thus, stroke prevention in AF patients is an
urgent healthcare and public-health concern.
Anticoagulant is the most important modifiable
factor to reduce stroke incidence in AF.7 An old
drug used to reduce stroke incidence, Warfarin, is a
long-established anticoagulant.8 However, since the
introduction of the non-vitamin K antagonist oral
anticoagulant (NOAC) in the 2010s, these new drugs
have changed the landscape for stroke prevention in
AF patients. Dabigatran was the first NOAC on the
market which was approved for stroke prevention in
patients with non-valvular AF by the US Food and Drug
Administration in 2010. Since then, 3 other NOACs
(rivaroxaban, apixaban, and edoxaban) are available in
many countries worldwide, including Hong Kong. Of
the 3, edoxaban, which was the last of them, became
registered in the Hong Kong Drug Office in 2016.
NOACs are not inferior to warfarin when used
for stroke prevention9,10, and some analyses of clinical
effectiveness suggests that they are actually preferable.11
NOACs are indicated to prevent stroke in patients
with non-valvular AF by both the European Society of
Cardiology (ESC) and the American Heart Association
(AHA) guidelines for patients with CHA2DS2-VASc
Score ≥1 in male, and ≥2 in female.1,12,13
However, there is still a great gap between
guidelines and the clinical utilisation rate of NOACs.
A nationwide study in Korea found that NOAC use
increased from 0% in 2002 to 14.% in 2016 in AF
patients.14 A Taiwan study of 181214 newly diagnosed
AF patients revealed that the NOAC use increased from
0% in 2008 to 26.0% in 2015.15
In order to promote the usage of the NOACs in AF
patients, dabigatran and apixaban were introduced into
our general out-patient clinics (GOPCs) of the Hospital
Authority (HA) in mid 2019. The prescription of these
NOACs was limited to patients with a high risk of
stroke whose CHA2DS2-VASc score was 5 or more. In
actuality, a number of AF patients still refused NOACs,
even through they are eligible for its use. Is this refusal
related to the problem of patient affordability? What is
the utilisation rate of NOAC in the GOPC setting?
So far, there is no study on the utilisation rate of
NOAC in the GOPC setting and no local data on the
associated factors for NOAC refusal. Therefore, this
study was conducted to evaluate the utilisation rate of
NOAC, and the factors independently associated with
NOAC refusal in AF patients, aiming at locating the
barriers to optimal NOAC use in the GOPC setting.
Method
Design and setting of the study
This was a cross-sectional study. A quantitative
method was selected because this approach can
objectively reflect the facts.
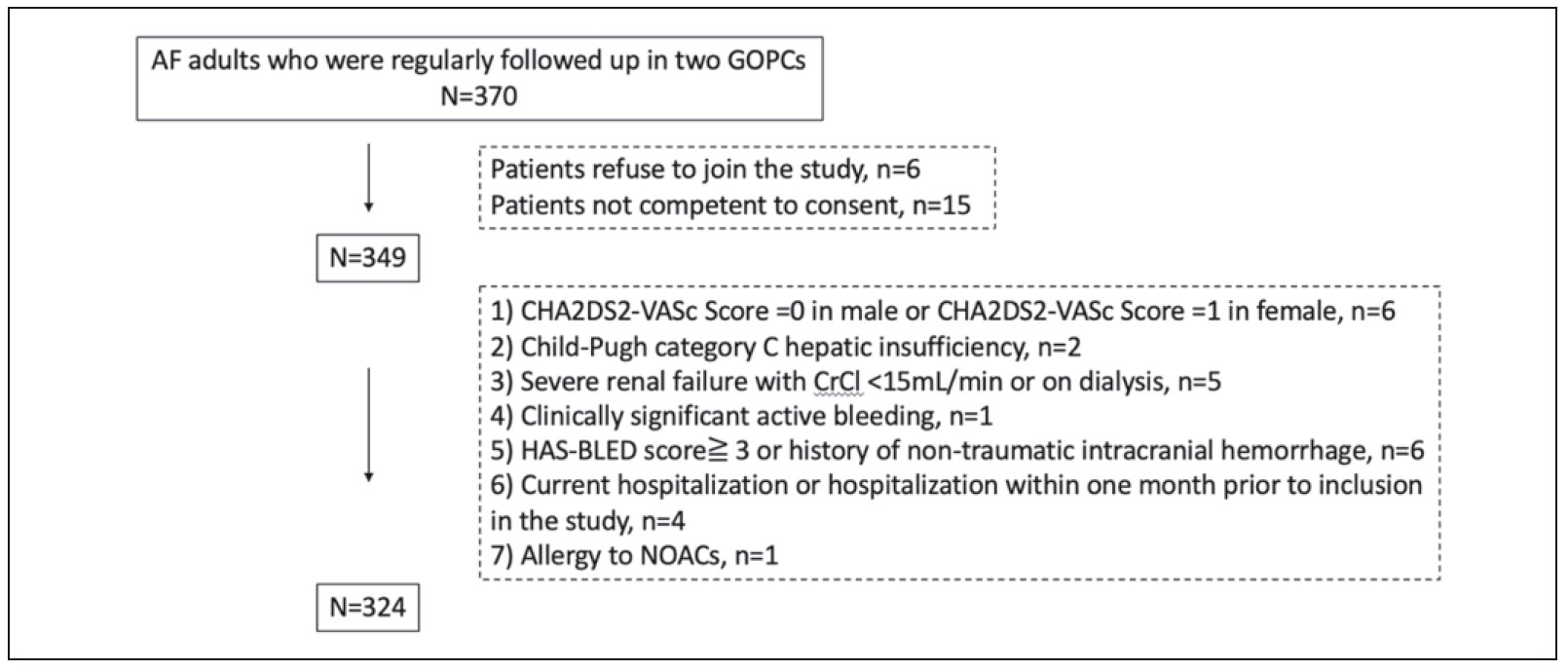
This study was conducted in two GOPCs in Tai
Po, with ethics approval by the Local Ethics Committee
(CREC Ref. No. 2019.516). The flowchart in
Figure
1
illustrated the patients’ enrolment in the study. A list
of patients with International Classification of Primary
Care (ICPC) coding of AF(K78) was retrieved from the
Clinical Data Analysis and Reporting System (CDARS)
of HA. All AF patients who were regularly followed up
in participating clinics were invited to attend the
Atrial
Fibrillation Clinic
(AFC). Patients aged older than 18
years were recruited during initial visit in November
2019 to December 2019. A follow-up visit was arranged
3 months after initial visit to review their option of
NOAC use and compliance. Study subjects were seen by
the principal investigator in the AFC, with active review
of the clinical condition and discussion of the NOACs
options. The suggestions of NOACs followed stroke
prevention guidelines recommended by the European
Society of Cardiology (ESC) 2016 and the American
Heart Association (AHA) 2014 and 2019.1,7,13 Patients
on warfarin were mainly followed up in Specialist Outpatient
Clinics (SOPDs). Hence, we do not initiate
warfarin in our GOPCs. Informed consent was also
obtained from the study subjects before enrolment into
the study.
Figure 1:
Flowchart of patients enrolled in the study
Patient selection
Patients having one or more of the following were
excluded:
(i) Males with CHA2DS2-VASc Score = 0, and
females with CHA2DS2-VASc Score = 1
(ii) Patients with moderate-to-severe mitral stenosis
(iii) Prosthetic valve or valve repair
(iv) Child-Pugh category C hepatic insufficiency.
(v) Severe renal failure with CrCl < 15mL/min or on dialysis
(vi) Clinically significant active bleeding
(vii) HAS-BLED score16 ≧ 3 or history of non-traumatic
intracranial haemorrhage
(viii) Pregnancy or breastfeeding mother
(ix) All Current hospitalisation or hospitalisation
within one month prior to inclusion in the study.
(x) Allergy to NOACs
(xi) Those who refuse to join the study, or not competent
to consent
Data collection
Baseline demographic and clinical data was
collected and recorded via a questionnaire and a review
of the medical records in the Hospital Authority’s
Clinical Management System (CMS).
The following data were collected (1)
sociodemographic information including age, sex,
education level, living condition, marital status,
financial strain, employment status; (2) drinking and
smoking habit; (3) any sponsor for NOACs; (4) duration
of atrial fibrillation; (5) CHA2DS2-VASc component
details; (6) NOAC use.
Financial strain data was collected instead of
income as it was a more powerful control variable than
income.17 The measure of financial strain was based
on four items: three items asked respondents whether
they had enough money to pay for their needs in food,
in medical services, and daily expenses, using a three-point
scale ranging from 1 = enough, to 3 = not enough.
The fourth question asked respondents to rate how
difficult it was for them to pay their monthly bill using
a four point scale, ranging from 1 = not difficult at all,
to 4 = very difficult. A sum of the scores of these four
items was computed, yielding a range from 4-13, with
high scores indicating greater strain.18
Drinker was defined as more than 7 units (for
women) or 14 units (for men) of alcohol in a week.
Smoker was defined as active smoking or smoking
cessation less than 1 year.
Sponsor for NOAC was defined as (a) CHA2DS2-
VASc score ≧5 hence no extra charge for NOAC in
GOPC, or (b) Civil service eligible persons with full
reimbursement of NOAC, or (c) Full reimbursement of
NOAC by insurance.
CHA2DS2-VASc score was calculated according
to the AHA guideline13: congestive heart failure,
hypertension, age ≥75 years (doubled), diabetes
mellitus, prior stroke or transient ischemic attack or thromboembolism (doubled), vascular disease, age 65 to
74 years, sex category, with theoretical score range 0–9.
Outcome variable
NOAC refusal group was defined as patient
refusing to take any NOAC during the consultation in
follow up visit. NOAC non-refusal group was defined
as those who used NOAC for at least 3 months and was
willing to continue NOAC during follow up visit.
NOAC refusal group was coded as “1 = refuse” and
“0 = not refuse” for the logistic model.
Statistical analysis
SPSS 24.0 software (SPSS, Chicago, IL, U.S.A.) was
used for analysis of data. Continuous data are presented
as mean ± standard deviation, and categorical data are
shown as number and percentage. Chi-square test was
used to compare categorical variables. For continuous
variables, independent T-test was used for comparing two
groups. Data was compared between the NOAC refusal
group and non-refusal group. Binary logistic regression
analysis was performed to identify factors significantly
associated with the refusal of NOACs. Adequate subject
number was based on 10 events per variable (EPV) in
logistic regression analysis.19 A P-value of less than 0.05
was considered to be statistically significant.
Results
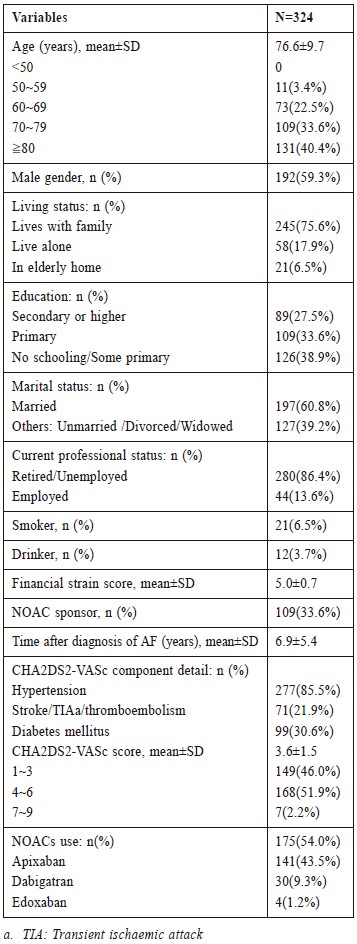
(A) Study population
A total of 324 AF patients from two GOPCs
were recruited. Average age was 76.6±9.7 years, and
192(59.3%) were male. Baseline demographic data,
clinical characteristics, and use of NOACs are shown in
Table 1. Among recruited patients, no one was younger
than 50 years old. The majority of patients (96.6%)
were older than 60 years old and most of the patients
had primary school education or below. 215(66.4%)
patients had no sponsor for NOACs. 109(33.6%)
patients had sponsor for NOACs and 21 patients among
them refused NOACs use. 277 patients (85.5%) had a
history of hypertension while 99(30.6%) patients had a
history of diabetes mellitus. Average CHA2DS2-VASc
score was 3.6±1.5 in this population.
(B) Utilisation rate of NOAC
Usage of NOACs were by 175(54.0%) patients.
Apixaban was 141 patients, (80.6% of all NOAC user)
(Table 1).
Table 1:
Baseline demographic and clinical characteristics
of the study population, and NOAC taken by the
study population.
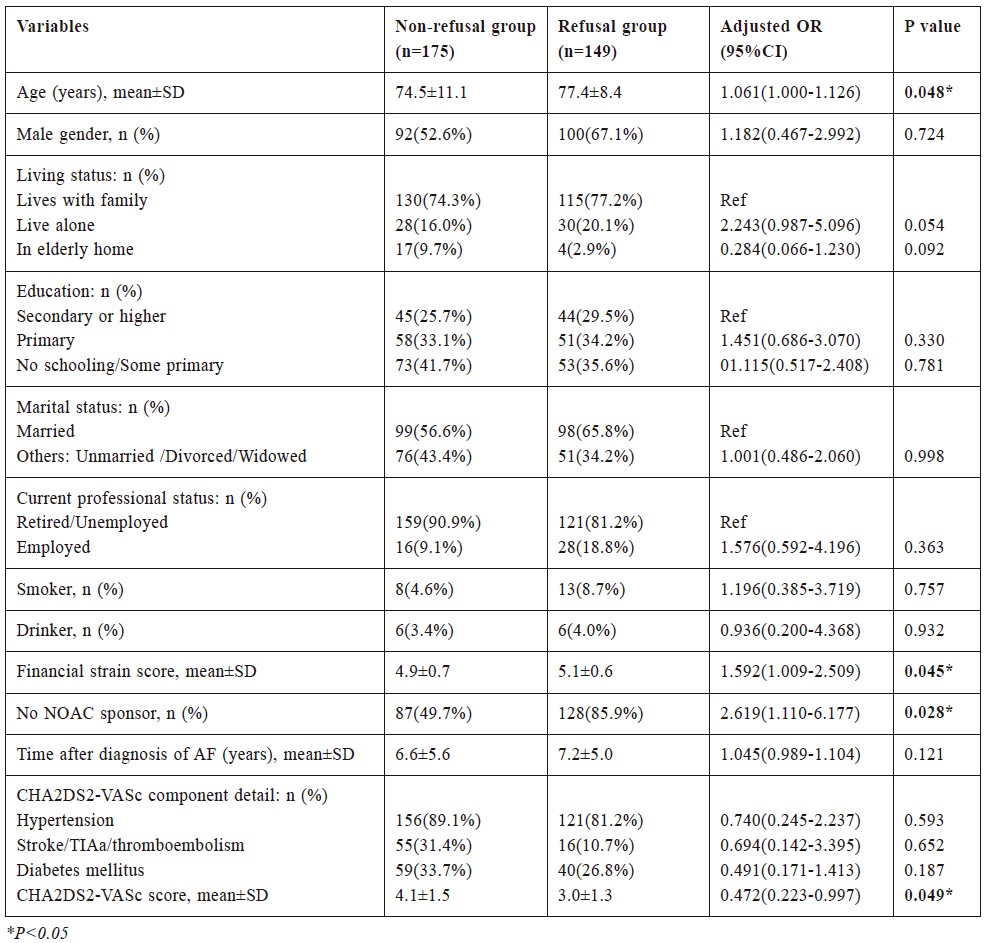
(C) Associated factors of NOAC refusal
Comparisons of data between NOAC refusal group
and non-refusal group were shown in Table 2.
Multivariate binary logistic regression analysis
revealed that older age (Adjusted OR 1.061, CI 1.000-
1.126, P=0.048), higher financial strain score (Adjusted
OR 1.592, CI 1.009-2.509, P=0.045), no sponsor for
NOAC (Adjusted OR 2.619, CI 1.110-6.177, P=0.028)
and lower CHA2DS2-VASc score (Adjusted OR 0.472,
CI 0.223-0.997, P=0.049) were independent associated
factors for NOAC refusal in AF adults.
Discussion
This study was conducted in AF patients who were
regularly followed-up in two GOPCs in Hong Kong.
All of the recruited patients were eligible for the use of
NOACs. The utilisation rate of NOAC in this population
was 54%, with room for improvement.
Overseas studies showed great disparity of NOAC
utilisation rate according to study time, region, and
type of institute. A Taiwan study with 181,214 newly
diagnosed AF patients revealed that the NOAC use
increased from 0% in 2008 to 26.0% in 2015.15 A study of 888,540 AF patients in Korea found that the
usage rate of NOAC was significantly different among
different medical systems from 37.2% at the tertiary
referral hospital and 5.5% at nursing or public health
centers.14 Another nationwide study in Korea showed
the proportions of prescribed NOACs to total oral
anticoagulants were 5.1%, 36.2%, and 60.8% in 2014,
2015, and 2016, respectively.20 A primary care study
in UK between June 2012 and June 2014 revealed
that 53% AF patients who were not on anticoagulation
agreed to start NOAC.21
Table 2:
Binary logistic regression analysis for factors associated with refusal of NOAC in AF patients
It was difficult to directly compare our NOAC
utilisation rate with previous studies as some of
them included warfarinised cases in their study
population14,15,21 and some of them aimed to analyse
the NOAC utilisation rate among all the anticoagulant
users.20 We could calculate the NOAC utilisation rate in
AF patients according to the data of the Taiwan study
to be 28.8% in 201515, which was lower than our data.
However, our data collection started in 2019 and a
higher utilisation rate was not surprising. Overall, this
study and overseas studies showed that there was still a
great gap between guidelines and the clinical utilisation
rate of NOAC.
Another aim of this study was intended to identify the
associated factors of NOAC refusal in the GOPC setting.
Our result showed that the lack of NOAC sponsor
and higher financial strain score were significantly
associated with NOAC refusal. NOACs were introduced
to GOPC in Hong Kong by the Hospital Authority in
mid 2019. However, the prescription was only limited
to patients with a high risk of stroke whose CHA2DS2-
VASc scores were 5 or above. Eligible patients can
be prescribed NOACs in the GOPCs without extra
cost after consultation. The non-eligible patients can
purchase NOACs in the community pharmacy with
a prescription issued by the GOPC doctor (they had
to pay HK$600 to HK$1500 per month according to
the type of NOAC and dosage). Obviously, for these
patients whose CHA2DS2-VAS2 scores were below 5,
affordability had a direct impact on their NOAC use,
and our findings shared similarities with other studies.
The previous studies demonstrated how health
policy and insurance influenced the use of NOACs.
In Korea, the policy of health insurance coverage for
NOACs was revised in July 2015 to allow a broader
coverage, and a study showed a significant growth
rate of NOAC prescription after this was introduced.20
Another nationwide study in Korea about newly
diagnosed AF patient revealed that partial and full
reimbursement of NOAC were independently associated
with higher anticoagulant use.14 There was a study on
associated factors for anticoagulants (including NOAC
and warfarin) use in 593 non-valvular atrial fibrillation
patients in China Jiangsu province, which showed self-paying
was negatively associated with anticoagulant
therapy in all patients.22
Based on the result of our study, a broader
sponsorship for NOACs in primary care was suggested
for the purpose of promoting the usage of NOAC.
Nevertheless, we also noticed in the group of 109
patients who were eligible for NOAC sponsorship,
21 patients (19.3%) still refused NOAC use, which
meant that patient affordability was not the only factor
associated with NOAC refusal. Unfortunately, a sample
size of 21 patients would not be sufficient to support
further quantitative analysis. Further research involving
a bigger sample size in this subgroup of patients would
be helpful to identify the barriers of NOAC use apart
from the money issue.
A previous study about NOAC adherence showed
education level and information about the disease could
affect the medication use.23 In our study, however,
patient’s education level was not related to NOAC
refusal based on the results of the multivariable analysis.
One of the possible explanations was that the patient’s
education level might not be equal to their knowledge
level which potentially influenced the decision of NOAC
use. Furthermore, the education level was difficult to be
graded in the elderly (mean age of our study subjects
was 76.6 years old) as most of them did not receive
formal education. Still, one had to bear in mind that
a multitude of factors besides the knowledge level of
patients potentially influenced the choice of medication.
Patients’ perspectives, perceptions and attitudes cannot
be well assessed in a quantitative study. Additional
qualitative research is needed to unravel and understand
these factors influencing NOAC use in patients.
Older age was an independent predictor of NOAC
refusal in our study. The elderly with AF, especially
those aged ≥75 years, are considered to have at least
a CHA2DS2-VASc score of 2.13 This population is
the group with the highest risk of stroke and the
worst prognosis, thus, oral anticoagulant is certainly
recommended. However, the data on the NOACs option
in the elderly was not sufficient in previous studies.
A small sample study among non-valvular AF patients
in China indicated that increasing age was negatively
associated with anticoagulant therapy (including NOAC
and warfarin).22 Plenty of studies about warfarin use in
the elderly showed that overestimation of the bleeding
risk and disadvantages associated with advanced age
are barriers to the prescription of oral anticoagulants
in the elderly.24 Therefore, the underutilisation of
NOAC in the elderly group might share similar reasons.
Other possible reasons include relatively lower mental
capacities to comprehend benefit and risk of NOAC
and difficulty to negotiate with family members before
decision making.
In general, the decision to prescribe NOAC in
the elderly is complicated. It requires not only to
balance the stroke risk and bleeding risk, but also
the need to consider the patient’s general health,
functional and cognitive ability, availability of a
caregiver, and patient’s attitude and preference towards
anticoagulation. More attention should be paid to this
group of elderly patients during consultation and setting
up of a special clinic with a multidisciplinary approach
to provide patient education, medication monitoring and
dosage adjustment might help.
Low CHA2DS2-VASc score was another associated
factor for NOAC refusal in our study. A study in
Thailand for AF patients aged ≥65 years showed
CHA2DS2-VASC score 1, to CHA2DS2-VASC score ≥2,
increases the rate of non-prescription of anticoagulant.25
However, the results of this study could not be compared
to our study which defined CHA2DS2-VASc score as
scale variable and excluded CHA2DS2-VASc Score
=0 in male or CHA2DS2-VASc Score =1 in female. A
Taiwanese study had similar results as our study. Patients
who were not on any antithrombotic therapy tended to
have lower CHA2DS2-VASc score (5.1±1.6) than those
taking antiplatelet agents (5.6±1.5) or warfarin (5.7±1.5).26
One explanation was that lower CHA2DS2-VASc score
implied lower risk of stroke and hence, lower cost
effectiveness of NOAC. However, the CHA2DS2-VASc
score could not predict the severity of stroke, whether
it’s major stroke or TIA. This study demonstrated a
barrier in initiating NOAC when patients have lower
CHA2DS2-VASc scores. This group of patients should
not be neglected, and methods of improving NOAC use
including educational programme should be put in place.
Strengths and limitations
There are strengths in this study. Firstly, this is the
first paper to quantify the use of NOACs in AF patients
in the Hong Kong GOPCs. There are important clinical
and policy implications. The use of NOACs in GOPCs
will become comparable and traceable. It also facilitates
policy holders in resource allocations and planning future
medical expenditures. Secondly, patient consultation was
conducted by the same principal investigator using the
same stroke prevention guideline throughout the study,
which could standardise the information that physicians
might deliver to patients.
However, there are several limitations in this study.
Firstly, study subjects were retrieved according to the
ICPC code, and there was a possibility of missing small
number of cases if the diagnosis of AF was not coded.
Secondly, this study lack generalisability as it was
carried out in two public primary care clinics. Warfarin
is another anticoagulant eligible for AF patients.
However, we do not keep warfarin patients in our GOPC
as they were mainly followed up in SOPD. Hence, there
was no warfarin user among our subjects. Finally, this
study just determined the associated factors but not
causative factors of NOAC refusal. Further research
is needed to identify major reasons of NOAC refusal,
and strategy to improve guideline attainment should be
developed and implemented.
Conclusion
With the increasing AF prevalence in our aging
population, it is important to identify the barriers of
NOAC use in AF patients. By employing a quantitative
design to investigate the NOAC utilisation rate, we
found that NOAC use in the two studied GOPC groups
of AF patients was 54%, which still leaves room for
improvement. This study identified four associated
factors of NOAC refusal: older age, higher financial
strain score, lack of sponsor for NOAC and lower
CHA2DS2-VASc score. The result highlighted the
importance of financial support to promote the usage
of the NOACs. Thus, future resources should be
focused on this high-risk group in order to reduce their
stroke risk and the subsequent financial burden due
to rehabilitation and hospitalisation. Further research
should focus on the subgroup of patients associated
with NOAC refusal, and strategy to improve guideline
attainment should be developed and implemented.
Acknowledgement
The author would like to thank our district
coordinator doctor and our clinic in-charge doctor
for their advice and supports on this study and the
AFC arrangement. Secondly, I would like to express
my wholehearted gratitude to the research committee
members for their valuable suggestion on my study
design. In addition, thanks all senior doctors in my clinic
for teaching me the proper writing of a research paper.
Funding/support
This research received no specific grant from any
funding agency in the public, commercial, or not-forprofit
sectors.
Conflict of interest
All authors have disclosed no conflicts of interest.
References
-
January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for
the management of patients with atrial fibrillation: a report of the American
College of Cardiology/American Heart Association Task Force on practice
guidelines and the Heart Rhythm Society. Circulation. 2014;130(23):e199-267.
-
Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development
of atrial fibrillation: the Framingham Heart Study. Circulation.
2004;110(9):1042-1046.
-
Ngai-yin Chan, Chi-chung Choy. Screening for atrial fibrillation in 13122 Hong
Kong citizens with smartphone electrocardiogram. Heart 2017;103:24–31
-
Chien KL, Su TC, Hsu HC, et al. Atrial fibrillation prevalence, incidence
and risk of stroke and all-cause death among Chinese. Int J Cardiol.
2010;139(2):173-180.
-
Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk
factor for stroke: the Framingham Study. Stroke. 1991;22(8):983-988.
-
Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the
risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946-952.
-
Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the
management of atrial fibrillation developed in collaboration with EACTS.
Eur Heart J. 2016;37(38):2893-2962.
-
Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention
of stroke associated with nonrheumatic atrial fibrillation. Veterans Affairs
Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. N Engl J
Med. 1992;327(20):1406-1412.
-
Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in
nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883-891.
-
Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in
patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093-2104.
-
Lopez-Lopez JA, Sterne JAC, Thom HHZ, et al. Oral anticoagulants for
prevention of stroke in atrial fibrillation: systematic review, network metaanalysis,
and cost effectiveness analysis. BMJ. 2017;359:j5058.
-
Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm
Association Practical Guide on the use of non-vitamin K antagonist
oral anticoagulants in patients with atrial fibrillation. Eur Heart J.
2018;39(16):1330-1393.
-
January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused
Update of the 2014 AHA/ACC/HRS Guideline for the Management
of Patients With Atrial Fibrillation: A Report of the American College
of Cardiology/American Heart Association Task Force on Clinical
Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol.
2019;74(1):104-132.
-
Yu HT, Yang PS, Hwang J, et al. Social Inequalities of Oral Anticoagulation
after the Introduction of Non-Vitamin K Antagonists in Patients with Atrial
Fibrillation. Korean Circ J. 2020;50(3):267-277.
-
Chao TF, Chiang CE, Lin YJ, et al. Evolving Changes of the Use of Oral
Anticoagulants and Outcomes in Patients With Newly Diagnosed Atrial
Fibrillation in Taiwan. Circulation. 2018;138(14):1485-1487.
-
Chiang CE, Okumura K, Zhang S, et al. 2017 consensus of the Asia Pacific
Heart Rhythm Society on stroke prevention in atrial fibrillation. J Arrhythm.
2017;33(4):345-367.
-
MENDES DE LEON CF, RAPP, S.S. & KASL, S.V. Financial strain and
symptoms of depression in a community sample of elderly men and women.
Journal of Aging and Health. 1994;4:448-468.
-
Chou KL, Chi I. Financial strain and life satisfaction in Hong Kong elderly
Chinese: moderating effect of life management strategies including selection,
optimization, and compensation. Aging Ment Health. 2002;6(2):172-177.
-
Peduzzi P, Concato J, Kemper E, et al. A simulation study of the number
of events per variable in logistic regression analysis. J Clin Epidemiol.
1996;49(12):1373-1379.
-
Ko YJ, Kim S, Park K, et al. Impact of the Health Insurance Coverage Policy
on Oral Anticoagulant Prescription among Patients with Atrial Fibrillation in
Korea from 2014 to 2016. J Korean Med Sci. 2018;33(23):e163.
-
Das M, Panter L, Wynn GJ, et al. Primary Care Atrial Fibrillation Service:
outcomes from consultant-led anticoagulation assessment clinics in the
primary care setting in the UK. BMJ Open. 2015;5(12):e009267.
-
Liu T, Yang HL, Gu L, et al. Current status and factors influencing oral
anticoagulant therapy among patients with non-valvular atrial fibrillation
in Jiangsu province, China: a multi-center, cross-sectional study. BMC
Cardiovasc Disord. 2020;20(1):22.
-
Emren SV, Senoz O, Bilgin M, et al. Drug Adherence in Patients With
Nonvalvular Atrial Fibrillation Taking Non-Vitamin K Antagonist Oral
Anticoagulants in Turkey: NOAC-TR. Clin Appl Thromb Hemost.
2018;24(3):525-531.
-
Wong CW. Anticoagulation for stroke prevention in elderly patients with
non-valvular atrial fibrillation: what are the obstacles? Hong Kong Med J.
2016;22(6):608-615.
-
Krittayaphong R, Phrommintikul A, Ngamjanyaporn P, et al. Rate of
anticoagulant use, and factors associated with not prescribing anticoagulant
in older Thai adults with non-valvular atrial fibrillation: A multicenter
registry. J Geriatr Cardiol. 2019;16(3):242-250.
-
Chao TF, Liu CJ, Lin YJ, et al. Oral Anticoagulation in Very Elderly
Patients With Atrial Fibrillation: A Nationwide Cohort Study. Circulation.
2018;138(1):37-47.
Liujing Chen,
LMCHK, FHKCFP, FRACGP, FHKAM (Family Medicine)
Associate Consultant,
Department of Family Medicine, New Territories East Cluster, Hospital Authority
Chik-Pui Lee,
MBChB (CUHK), FHKCFP, FRACGP, FHKAM (Family Medicine)
Associate Consultant,
Department of Family Medicine, New Territories East Cluster, Hospital Authority
Lit-Ping Chan,
MBBS (HK), FHKCFP, FHKAM (Family Medicine)
Associate Consultant,
Department of Family Medicine, New Territories East Cluster, Hospital Authority
Eric MT Hui,
MBBS (HK), FHKCFP, FRACGP, FHKAM (Family Medicine)
Consultant,
Department of Family Medicine, New Territories East Cluster, Hospital Authority
Maria KW Leung,
MBBS (Lond), FRACGP, FHKCFP, FHKAM (Family Medicine)
Consultant,
Department of Family Medicine, New Territories East Cluster, Hospital Authority
Correspondence to:
Dr. Liujing Chen, Lek Yuen General Out-patient Clinic, G/F,
9 Lek Yuen Street, Shatin, N.T., Hong Kong SAR.
E-mail: cl802@ha.org.hk
|