|
June 2015, Volume 37, No. 1
|
Original Article
|
|
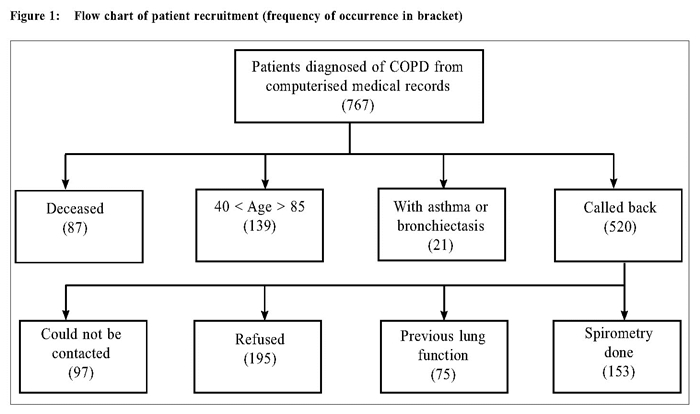
A study on COPD in primary care: comparing correctly and incorrectly diagnosed patientsMS U 余美媟,WK Leong 梁永權,YT Wun 溫煜讚,SF Tse 謝師輝 HK Pract 2015;37:14-22 Summary Objective: Chronic obstructive pulmonary disease (COPD) can be frequently misdiagnosed in primary care if diagnosis is based on respiratory symptoms and without the availability of spirometry. Little is known about patients who were correctly or incorrectly labelled to have COPD. Design: A cross-sectional study. Subjects: Patients aged 40-85 years previously diagnosed with COPD and without other concomitant lower respiratory tract diseases and has had no previous lung function tests. Main outcome measures: COPD Assessment Test (CAT), and office spirometry. Results: Among 152 patients with spirometry performed, 83 (54.6%) had persistent airflow limitation (FEV1/FVC < 0.7), and hence had COPD by the GOLD definition. COPD patients were significantly associated with a BMI < 24, CAT score ≥ 10, and had history of quitting smoking. The misclassified patients were more likely to have dyslipidaemia, and were significantly less likely to be prescribed bronchodilators or inhaled corticosteroids. Conclusion: Spirometry is essential for the diagnosis of COPD. A high CAT score clinically supports the diagnosis. Keywords: COPD, spirometer, COPD Assessment Test (CAT), primary care, Macau 摘要 目的:慢性阻塞性肺疾病(COPD)可以常常於基層醫療中被誤診。原因在於診斷是依據呼吸道症狀以及沒有肺功能測試的幫助。對於被錯誤地標籤上患有慢性阻塞性肺病患 者的特點則鮮為人知。 設計:橫切面式研究。 研究對象:年齡介於40-85歲被診斷為慢性阻塞性肺病患者並沒有其他下呼吸道的疾病或以前曾接受肺功能測試。 主要測量內容:慢性阻塞性肺病評估測試(CAT)和辦公室肺功能測試。結果:在152名病人中進行了肺功能檢查,83人(54.6%)有持續性氣流受限(FEV1/FVC < 0.7),因此合乎全球性行動計畫(GOLD)的慢性阻塞性肺病定義。慢性阻塞性肺病患者的顯著特點包括身高體重指數< 24,慢性阻塞性肺病評估測試分數 ≥ 10和已經戒除吸煙的病歷。被錯誤分類的患者更可能具有血脂異常問題,並顯著地較少接受支氣管擴張劑或吸入性類固醇治療。 結論:肺功能測試是慢性阻塞性肺病診斷的必要條件。高慢性阻塞性肺病評估測試分數有助臨床診斷。 主要詞彙:慢性阻塞性肺病,肺量計,慢性阻塞性肺病評估測試(CAT),基層醫療,澳門 Introduction Persistent limitation of airflow is the diagnostic criterion of chronic obstructive pulmonary disease (COPD) according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD).1 COPD should be a diagnosis based on lung function tests. However, spirometry is not often done in many primary care practices. 2-5 Some practices do not have spirometers. 6,7 An international study on primary care physicians from 12 territories revealed a gross under-utilisation of the spirometry test even though it may be readily accessible. 8 In some other studies, most primary care physicians diagnosed COPD based on working hypotheses, 9 or used the spirometer only in diagnostic difficulties. 10 When lung function tests are not used to confirm the diagnosis, misdiagnosis of COPD is common.10-13 Although misclassification of COPD had been widely shown in the past and many patients were affected, not much information about these patients are available. Some information could have also been conflicting. For example, Jones et al observed that the patients correctly classified to have COPD were more likely to have increased body mass index (BMI) than those misclassified, 14 but Walters et al observed the opposite. 12 COPD patients were found to have more co-morbid conditions than the misclassified patients in one study2 but have less co-morbidities in another. 13 Better understanding of these two groups of patients might help to improve the diagnostic accuracy, and hence the management of COPD even when spirometry might not be readily accessible. The clinical presentations of COPD are non-specific. Common clinical features including cigarette smoking, productive cough, sputum, dyspnoea, and wheezing, are all non-specific for persistent airflow limitation. Various combinations of respiratory symptoms were observed to increase the likelihood of airflow limitation3,15-18 but the combinations were inconsistent across different studies. Clinical questionnaires based on symptoms, though helpful for diagnosis, were limited by their relatively low positive predictive value.18 The assessment of functional limitation like the MRC (Medical Research Council) dyspnoea scale was suggested to have additional diagnostic value.17 But, due to the limited studies, the usefulness of such functional scales is uncertain. The COPD Assessment Test ( CAT ) is a self-administered functional scale designed to assist the primary care physicians to better understand the severity and impact on the life of COPD patients.1,19,20 The CAT has been validated and translated into different languages.21 The Chinese version is available at http://www.catestonline.org/english/index_ChinaTrad.htm. Using CAT, Raghavan et al detected 51 COPDs in 532 people, aged 40 years or above, randomly selected from the general population.22 Their observation suggests that CAT may help identify people at risk for COPD. However, their findings have not been verified nor replicated. Whether it helps in the clinical diagnosis of COPD is unknown. The aim of this study was to evaluate if clinical features, including the CAT, could help identify airway limitation among patients suspected of COPD. These factors might help the primary care physicians lessen the misclassification of COPD in the absence of spirometry. MethodsThis was a cross-sectional study. Subjects Patients aged 40-85 years from the public primary care health centres in Macau, assigned with the diagnostic code of R97 (the International Classification for Primary Care for COPD) between 01/01/2005 and 31/1/2012 were identified in the central computerised medical record system and called back. Spirometers were not available in the studied health centres during this period. The exclusion criteria were (a) patients with other active lung diseases such as confirmed asthma, tuberculosis, bronchiectasis, recent pneumothorax, (b) patients unsuitable for forceful expiration such as recent eye or abdominal surgery, recent heart attack or stroke, unstable angina, (c) patients with diagnosis of COPD by previous lung function tests, and (d) patient with communication difficulties. After informed written consent, they completed a structured questionnaire and then blew into an office spirometer (MIR Spirolab III). Data Collection Data obtained from the structured questionnaire include socio-demographic variables (sex, age, occupation, levels of education), tobacco exposure (pack-years, current cigarettes per day, ages of starting and quitting), and respiratory symptoms (chronic cough, sputum, dyspnoea, wheeze) before the diagnosis of COPD, frequency of visits to the emergency department in the past year for respiratory complaints, and the CAT. Current medications and co-morbid conditions (e.g. old cerebrovascular diseases, diabetes mellitus, dyslipidaemia, hypertension, deafness, or osteoporosis) were retrieved from the medical records. Spirometry For the spirometry, the patient inhaled 400μg of salbutamol from a metered-dose inhaler with a spacer. After 15 - 20 minutes, they blew into the spirometer thrice in a sitting position and with full effort. The best result was taken. The FEV1/ FVC ratio < 0.70 was taken as airflow limitation according to the GOLD criteria. COPD was defined as the presence of chronic respiratory symptoms plus persistent airflow limitation. Two of the authors (MSU and WKL) supervised the spirometry. The body height and weight were taken for the body mass index (BMI). Statistical method Variables were described in proportions or means with standard deviations (SD). BMI and CAT scores were separated into groups with BMI ≥ 24 (the cutoff point for overweight in Chinese (not Hong Kong residents) by the World Health Organisation and below BMI < 18.5 (the cut-off point for underweight); and CAT score ≥ 10 (moderate to severe impairment) or otherwise. The chi-squared test (corrected for continuity in 2 x 2 tables), Student’s t-test, and ANOVA were used to test the association between variables. For the variables with significant association, logistic regression was then used to test for the adjusted effect on airflow limitation. No interaction was tested, however. P< 0.05 was taken as statistically significant. Results Patients recruitment There were 767 patients with the diagnosis of COPD in the computerised medical record system. Of them, 138 patients were aged above 85 years and one was below 40; another 87 had died. A further 21 patients were excluded as they had also asthma or bronchiectasis. So, 520 patients were recalled, of whom 97 could not be contacted after three attempts, 195 refused, and 75 had previous lung function tests done. Finally 153 patients underwent spirometry (Figure 1). One patient failed to blow into the spirometer with satisfactory effort and was excluded from analysis.
Study population characteristics (Table 1) Spirometry was successfully performed for 152 patients, the majority of whom were males (76.3%) with a mean age of 67.8 years. 83 (54.6%) had persistent airflow limitation, among whom, 12 (14.5%) were in GOLD stage I, 34 (41.0%) stage II, 25 (30.1%) stage III, and 12 (14.5%) stage IV. There was no significant difference in age, sex, and education level (Table 1) between patients with and without persistent airflow limitation. Non-smokers were not free from COPD. The proportion of those ever-smoked was higher in those with airflow limitation than those never; but the difference was marginally insignificant with an odds ratio (OR) of 2.12 (95% confidence interval (CI) [0.94, 4.84]). The smoking pack-years and the distribution of heavy smokers were similar among the two groups. However, patients with airflow limitation were more likely to have quitted smoking (OR = 2.38, 95% CI [1.04, 5.48]). BMI was significantly different between patients with persistent airflow limitation and those with no limitation (Table 1). A BMI < 24 carried an OR for persistent airflow limitation of 3.64 (95% CI [1.73, 7.73]), with a sensitivity of 75.9% (95% CI [68.3%, 82.7%]), specificity of 53.6% (95% CI [44.5%, 61.8%]), and positive predictive value (PPV) of 66.3% (95% CI [59.7%, 72.2%]). A BMI < 18.5 carried an OR of 4.09 (95% CI [1.02, 18.98]), with sensitivity of 15.7% (95% CI [10.6%, 18.37%]), specificity of 95.7% (95% CI [89.6%, 98.8%]), and PPV of 81.3% (95% CI [55.1%, 95.0%]). BMI, however, was not correlated with the CAT score (R2=0.01).
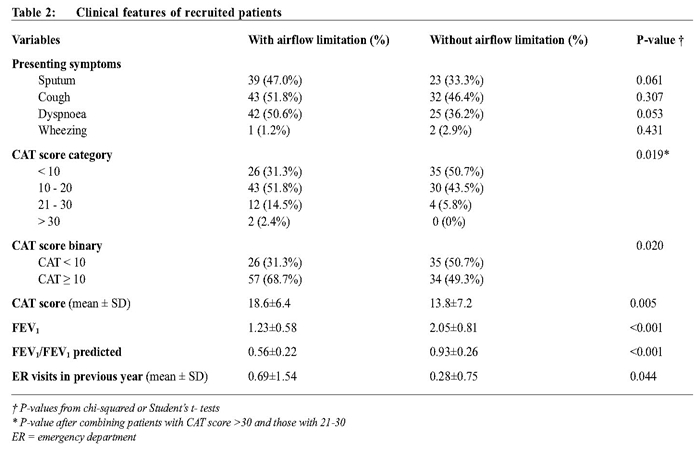
Clinical features The frequencies of respiratory symptoms (cough, sputum, dyspnoea, wheezing) were similar between the two groups of patients (Table 2). Patients with or without airflow limitation had similar occurrence of co-morbid conditions, e.g. ischemic heart disease, hypertension, stroke, or diabetes mellitus. However, significantly more patients without airflow limitation reported dyslipidaemia (21 out of 69 vs 14 out of 83, χ2 (1, n=152) = 3.91, P = 0.037). None of the recruited patients reported depression.
Patients with persistent airflow limitation were more likely to have visited the emergency department in the previous year and had higher CAT scores. Patients with CAT score ≥ 10 were much more likely to have airflow limitation, with an OR of 2.26 (95% CI [1.11, 4.63]), sensitivity of 68.7% (95% CI [61.0%, 75.9%]), specificity of 50.7% (95% CI [41.4%, 59.5%]), and PPV of 62.6% (95% CI [55.6%,69.3%]). Medications Apart from theophylline, there was markedly significant difference in bronchodilator prescription between the two groups of patients. Patients without airflow limitation were much less likely to receive them (Table 3). Although more than 80% of the patients with airflow limitation were of GOLD stage II or above, only 45.8% of them were prescribed inhaled corticosteroids. The expensive anticholinergics were rarely prescribed due to the limited resources in the health centres. Patients with persistent airway limitation No underweight patients were in GOLD stage II or worse, while CAT scores of some patients in stage I were ≥ 10 (Table 4). Among these patients, the CAT score was not correlated with smoking status (R2 = 0.01) or the BMI (R2 = 0.01). CAT score and BMI were factors independently associated with persistent airway limitation. Patients in stage IV had the highest proportion of current smokers though their CAT score were more likely to be ≥ 10. Only the mean BMI and the distribution of underweight reached statistical significance among patients with persistent airway limitation. Patients in stage IV had the lowest mean BMI and the highest proportion of being underweight. Regression model In the logistic regression model with BMI < 24, CAT score ≥ 10, smoking history, and visits to the emergency department as independent variables, visits to the emergency department were not significantly associated with persistent airflow limitation. The final logistic regression model showed that a BMI < 24 had the highest odds ratio (Table 5). When BMI < 18.5 replaced the factor BMI < 24, underweight carried an adjusted OR of 5.12 (95% CI [1.306, 20.080]).
Discussion In this study, 54.6% of patients previously diagnosed with COPD without spirometry were confirmed to have persistent airflow limitation (i.e. COPD) according to the GOLD criteria. Symptoms could not differentiate patients from having COPD or not. Misclassified patients were more likely to have dyslipidaemia; they were also less likely to have visited the emergency department in the past year, or been prescribed bronchodilator drugs. Correct COPD diagnosis was significantly associated with a BMI < 24 (more significantly with BMI < 18.5), former smoking, and a CAT score of 10 or above. In the absence of spirometry, misclassification of COPD would be inevitable. That 45.4% of patients in this study were misclassified appeared to be high. Similar rates were reported by some studies from primary care (49.8% in Greece,24 42.2% in Australia13). This is common because of non-compliance to COPD guidelines,8 which mandates diagnosing COPD with spirometry. Primary care physicians who are not ready or without direct access to spirometry can only make the diagnosis of COPD clinically, but the accuracy could not be assured. Prolonged cough, sputum, dyspnea and wheeze are common presenting symptoms of chronic bronchitis. However, patients with chronic bronchitis may have normal spirometry. GOLD does not include chronic bronchitis into the diagnosis of COPD and regards it as an independent entity that may precede or follow COPD. Nevertheless, the World Health Organisation states that “the more familiar terms ‘chronic bronchitis’ and ‘emphysema’ are no longer used, but are now included within the COPD diagnosis”.25 The American Lung Association also states that “these two conditions [chronic bronchitis and emphysema] together are commonly referred to as Chronic Obstructive Pulmonary Disease (COPD)”.26 It could be argued that some physicians might regard the two as different stages of the same chronic lung disorder and coded the diagnosis of chronic bronchitis/emphysema under COPD. Patients without persistent airflow limitation in this study were significantly less likely to be prescribed with long-acting bronchodilators or inhaled corticosteroids, compatible with the treatment of chronic bronchitis or emphysema. This is only a speculation but should be seriously considered to look into COPD misdiagnosis in future studies. We used the GOLD guidelines as it was the most updated one at the time of our study. It is not free from controversies, especially using the fixed ratio of FEV1/ FVC < 0.70 instead of an age-sex related lower limit of normal as the diagnostic criterion of airflow limitation. GOLD defines COPD as “characterised by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases”. This definition excludes many patients of chronic bronchitis or emphysema. But GOLD also states that “a clinical diagnosis of COPD should be considered in any patient who has dyspnoea, chronic cough or sputum production, and a history of exposure to risk factors for the disease”. Such “clinical diagnosis” would include chronic bronchitis and emphysema. However, GOLD further states that “the presence of a post-bronchodilator FEV1/FVC < 0.70 confirms the presence of persistent airflow limitation and thus of COPD”. Some clinicians might take that a diagnosis of COPD could be made on clinical symptoms, unaware of the fact that the clinical diagnosis needs confirmation. A clinical diagnosis that needs confirmation is not the final diagnosis. Spirometry should therefore be done for the diagnosis of COPD. Smoking was very common in patients with COPD in this study though marginally short of statistical significance in differentiating airflow limitation (Table 1). From statistical analysis, being an ex-smoker was an independent factor associated with persistent airway limitation. We are not aware of other studies with similar finding of ex-smokers in COPD. A plausible explanation is that the COPD patients were more motivated to quit smoking due to their disability though this study was not set to test if smoking cessation preceded or followed the diagnosis. But there is no significant association between smoking history and the CAT score (the subjective severity of the disease). Furthermore, 38.2% of the COPD patients, and half of those in stage IV, were still smoking – far too high for these patients. Smoking by COPD patients must be a complex problem; their motivation and success in quitting smoking should be further researched. Existing literature relating BMI and COPD is conflicting.12,14,27 Underweight (BMI < 18.5) was shown not only to be common in COPD patients28 but also a risk factor for COPD. 29 This study observed that COPD patients were more likely to have low or normal BMI. It also showed that overweight was significantly associated with the misclassification of COPD, consistent with the conclusion by Walters et al. 12 Like smoking habit, the status of BMI before the diagnosis of COPD was not included in the design of this study. For this study, BMI was a significant factor but uncertain to be a predicting factor. In this study, a CAT score ≥ 10 was significantly associated with persistent airflow limitation even after controlling for smoking and BMI (Table 5). More importantly, such a high score was present early even in stage I patients (Table 4). This supports the finding by Raghavan et al that the CAT could identify people at risk of COPD. 22 Although GOLD used CAT to assess the severity and management of COPD, this tool (or other functional scales) might be helpful in the diagnosis of COPD in the absence of spirometry. Limitations This study recruited patients diagnosed and recorded in the computerised system to have COPD. It had no information of patients with COPD who were not diagnosed or not recorded. It is uncertain if the under-diagnosed COPD patients had their specific characteristics. At its best, this study observed that respiratory symptoms would lead to over-diagnosis of COPD; its findings did not apply to under-diagnosis. Another limitation is the sample size. This study recruited 152 patients, 83 of whom had airflow limitation. These sample sizes were adequate to detect large differences between two groups at power = 0.80 and α = 0.05, the “large effect size” as depicted by Cohen30 but not the medium one (for which 177 participants in each group would be required). So, if the observed difference between patients with and without airflow limitation was marginally insignificant, this might be due to the limited sample sizes rather than a true non-difference. Furthermore, of the 293 patients eligible for spirometry (after exclusion criteria), 152 valid tests were finally done; a response rate of 52.0%. Unfortunately non-respondents were not traced for comparison with the respondents. Conclusion Spirometry is essential for the diagnosis of COPD; respiratory symptoms alone would lead to incorrect diagnosis. Patients correctly diagnosed with COPD were more likely to have a low BMI, high CAT score and quitted smoking. Functional scales like the CAT should always be deployed in the initial assessment of the disease.
Funding This study received no funding from any source. Ethical approval was granted by the Health Bureau of Macau. Acknowledgement All doctors took part in the design of the study. MSU and WKL collected the data. YTW did the statistical analysis. MSU and YTW drafted the manuscript. SFT coordinated and supervised the study. All authors read and approved the final version of the manuscript. MS U, MB Correspondence to: Dr MS U, Trav. Inacio Baptista, No. 2, Edf. San Tou Kok, Bloco 2, r/c, Macau SAR, China. e-mail: drevaumacau@yahoo.com.hk References
|
||